Phage Phi X174 lytic cell protein E and application thereof
A bacteriophage Ф, X174 technology, applied in the field of genetic engineering, can solve the problems of increasing the cost of conjugated linoleic acid, etc., and achieve the effect of simple operation, good application prospect and easy use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
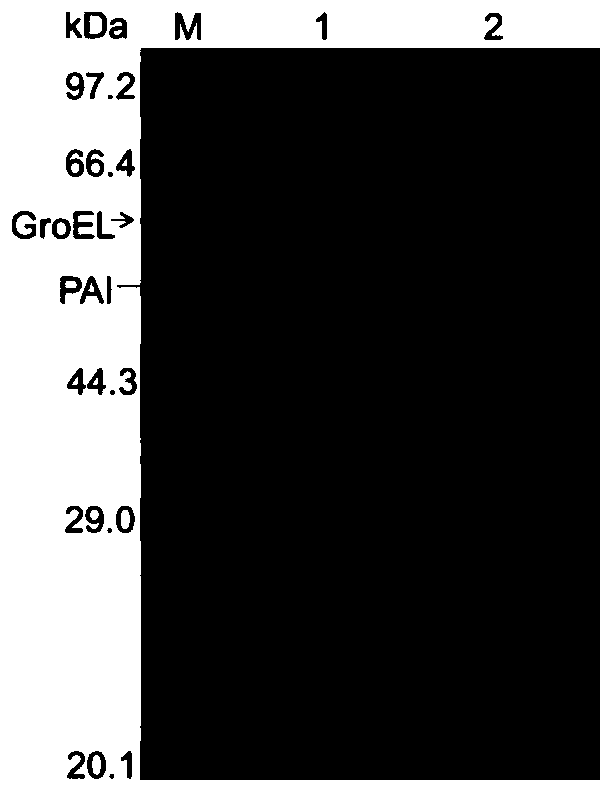
[0031] Pick a single colony from a fresh plate containing E.coli (PAI / GroELS), and culture it in LB medium with kanamycin and chloramphenicol at a final concentration of 100 μg / ml, and culture at 37°C, 200rpm OD 600 = 0.4-0.6, take it out from the shaker, and centrifuge in ice bath for 30 minutes. With 0.1mol / L CaCl 2 Wash the cells 3 times. Add pBV220-E plasmid to competent cells, heat shock in 42°C water bath for 90s after ice bath for 30min, add 1ml of LB medium, incubate at 37°C for 1h, spread the corresponding resistance plate, and pick the correct one after it grows out. Transformants were obtained to obtain recombinant E. coli (PAI / GroELS / E) containing corresponding plasmids.
Embodiment 2
[0033] Pick a single colony of recombinant bacteria E.coli (PAI / GroELS / E) from the plate in the LB medium containing the corresponding antibiotics, cultivate it overnight as a seed solution, and inoculate it in 50 mL of fermentation medium with an inoculum of 2%. , while adding the final concentration of 4.0mg / ml L - Arabinose induces chaperone protein expression. Cultivate the bacteria concentration to OD 600 When =1.5, add IPTG to a final concentration of 0.1 mmol, lower the temperature to 20°C and culture at 200rpm for 20 hours, raise the temperature to 42°C and maintain for 3 hours to induce lysis of recombinant E. coli cells.
Embodiment 3
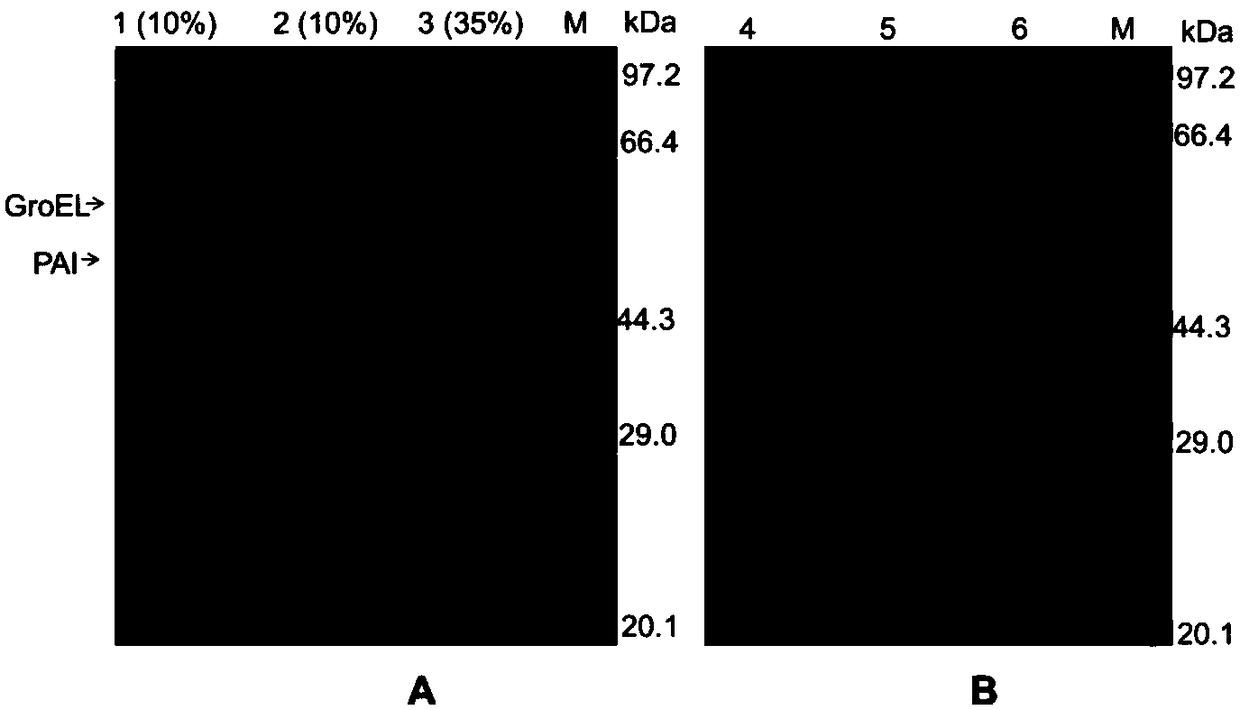
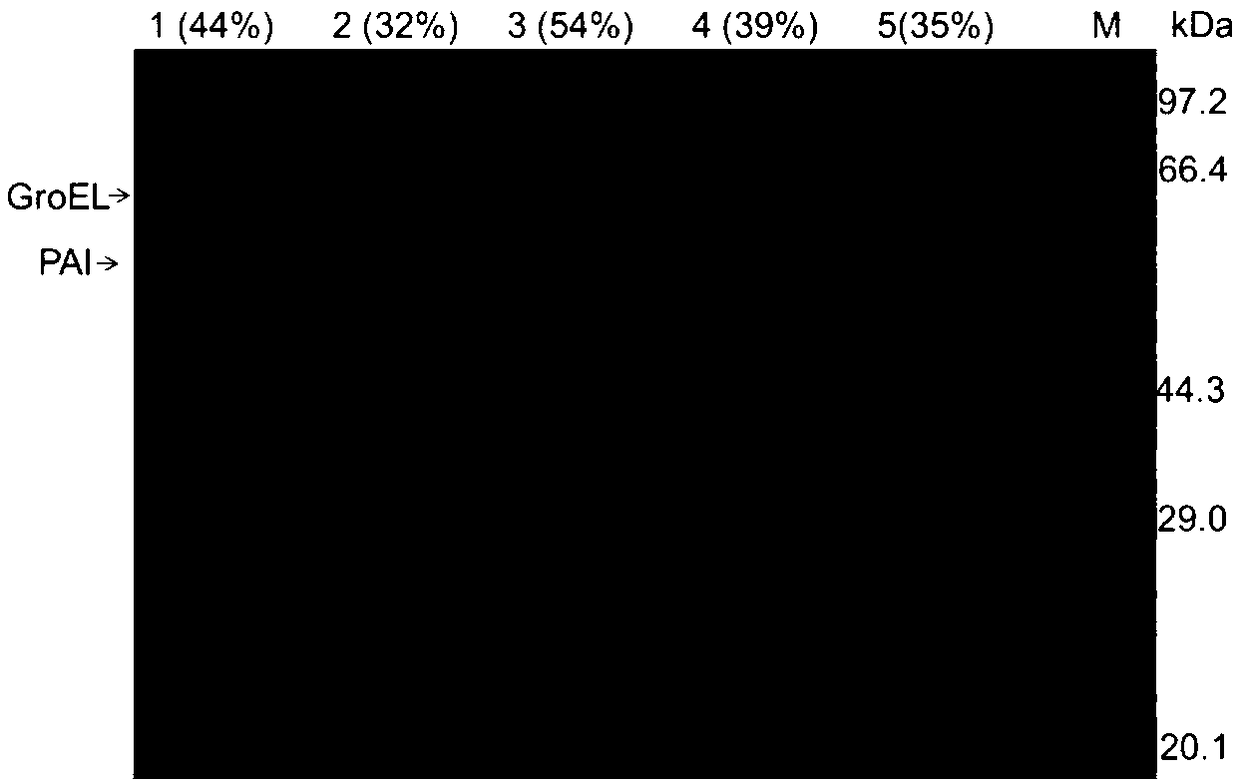
[0035]The induced lysed and intact cells were collected at 8000×g, 4°C, and centrifuged for 5 minutes, and the same amount of cells were suspended in hypertonic 30% glycerol solution, isotonic saline solution and hypotonic double-distilled solution, and placed in an ice-water bath Shake for 2h. The supernatant was collected by centrifugation at 8000×g at 4°C for 10 min, and the enzyme activity of the supernatant was determined. PAI release rate=released enzyme activity÷intracellular enzyme activity (measured value of enzyme activity after wall breaking by ultrasound)×100%. EDTA, Tween-20, sodium deoxycholate (DOC), and Triton X-100 were added to double-distilled water to a final concentration of 100 mM, 0.5%, 0.2%, and 0.5% to promote the release of PAI.
[0036] The DOC concentration was optimized, and the results showed that the addition of 0.005-0.1% DOC had little effect on the release rate of PAI, and the release rate was 35-39%. The PAI release rate increased significan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com