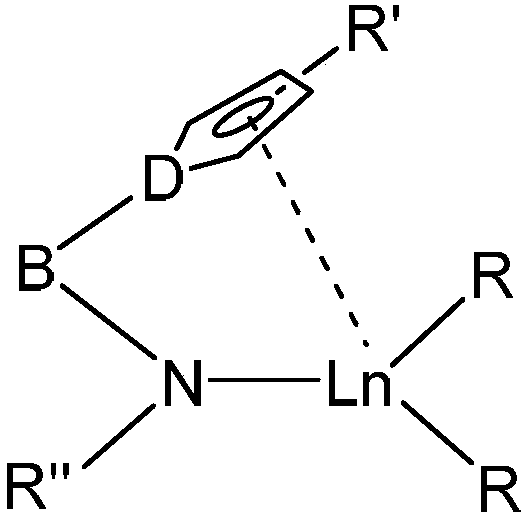
Rare earth catalyst and preparation method thereof and styrene syndiotactic polymerization method
A rare earth catalyst and catalyst technology, which are applied in the directions of organic chemistry methods, chemical instruments and methods, compounds of Group 4/14 elements of the periodic table, etc., can solve the problems of high cost of catalysts and limited application, and achieve simple and feasible preparation methods. The effect of cost reduction and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
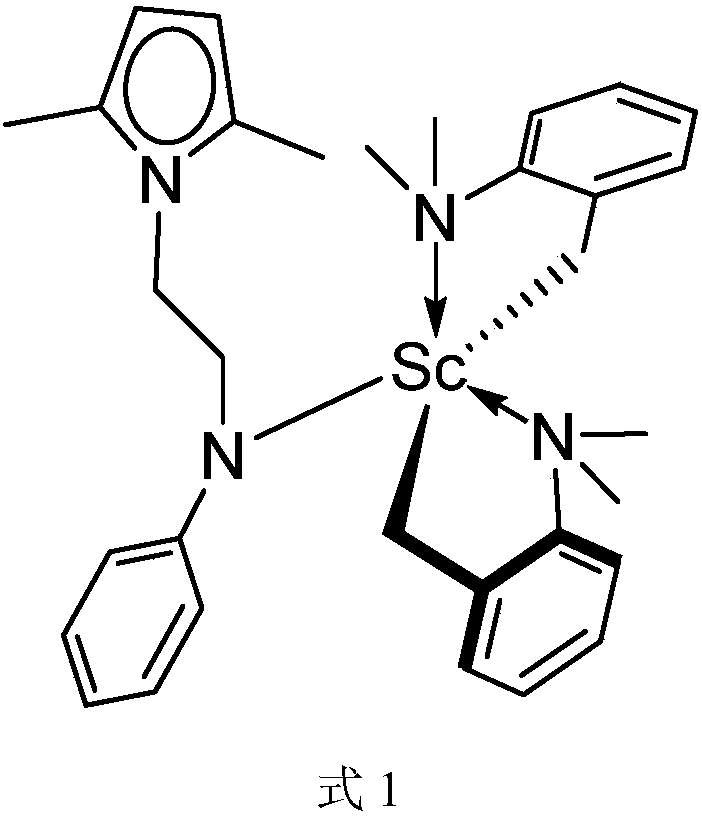
[0036] The mononuclear rare earth catalyst in this example is an ethyl-bridged 2,5-dimethylpyrrole aniline dibenzyl scandium compound (2,5-Me 2 C 4 h 2 NCH 2 CH 2 NC 6 h 5 )Sc(CH 2 C 6 h 4 NMe 2 -o) 2 ; Hereinafter referred to as catalyst 1, its structural formula is as shown in formula 1:
[0037]
[0038] The concrete preparation method of this catalyst is as follows:
[0039] In the glove box, the Sc(CH 2 C 6 h 4 NMe 2 -o) 3 (224mg, 0.5mmol) was dissolved in 5mL of toluene, 2,5-Me 2 C 4 h 2 NCH 2 CH 2 NHC 6 h 5 (107 mg, 0.5 mmol) was dissolved in 5 mL of toluene, and the two solutions were mixed to obtain a yellow clear liquid. After reacting at room temperature for 15 hours, the solvent was sucked dry, and the residual yellow oil was washed with hexane (3 times, the amount of hexane was 5 mL each time). Then it was sucked dry under reduced pressure to obtain 0.23 g of light yellow powder, and the calculated molar yield was 85%.
[0040] The calc...
Embodiment 2
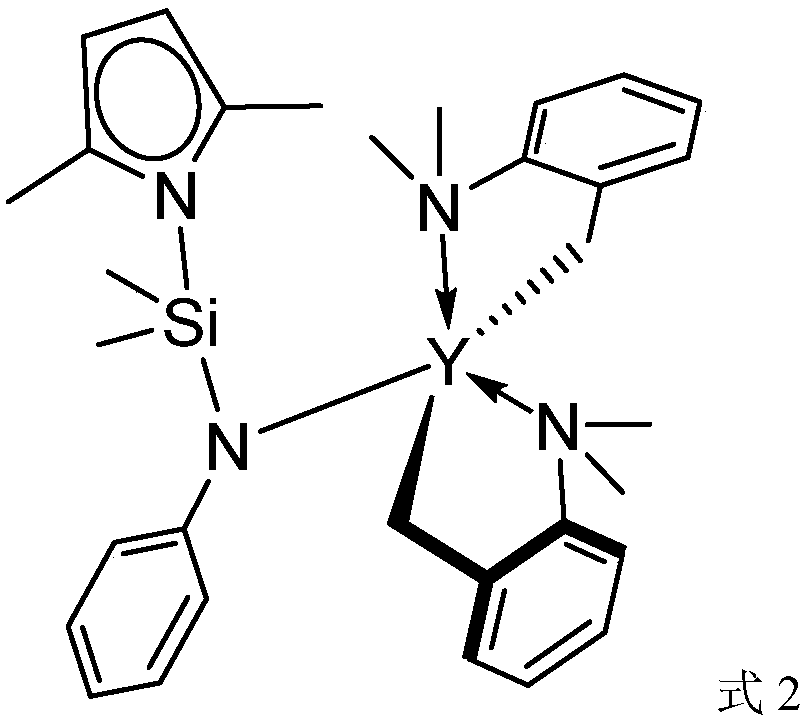
[0046] Catalyst (hereinafter referred to as catalyst 2) in the present embodiment, its structure is as shown in formula 2:
[0047]
[0048] The concrete preparation method of this catalyst is as follows:
[0049] In the glove box, the Y(CH 2 C 6 h 4 NMe 2 -o) 3 (246mg, 0.5mmol) was dissolved in 5mL of toluene, 2,5-Me 2 C 4 h 2 NSiMe 2 NHC 6 h 5 (122 mg, 0.5 mmol) was dissolved in 5 mL of toluene, and the two solutions were mixed in a Schlenk bottle to obtain a yellow clear liquid. The reaction bottle was removed from the glove box, and reacted in a pre-prepared 50°C oil bath for 5 hours, then the solvent was drained, and the residual yellow oil was washed with hexane (washing 3 times, the amount of hexane was 5 mL each time). Then it was sucked dry under reduced pressure, and recrystallized in a mixed solution of toluene and hexane to obtain 0.22 g of colorless crystals, and the calculated molar yield was 71%.
[0050] Characterization data for dimethylsilyl br...
Embodiment 3
[0054] The rare earth catalyst (hereinafter referred to as catalyst 3) of this embodiment is dimethylsilylmethylene bridged 2,5-dimethylpyrrole p-chloroaniline dibenzyl lanthanum compound (2,5-Me 2 C 4 h 2 NCH 2 SiMe 2 NC 6 h 4 Cl-p)La(CH 2 C 6 h 4 NMe 2 -o) 2 ; Its structure is as shown in formula 3:
[0055]
[0056] The concrete preparation method of this catalyst is as follows:
[0057] In the glove box, La(CH2 C 6 h 4 NMe 2 -o) 3 (542mg, 1.0mmol) was dissolved in 10mL of toluene, 2,5-Me 2 C 4 h 2 NCH 2 SiMe 2 NHC 6 h 4 (p-Cl) (293 mg, 1.0 mmol) was dissolved in 10 mL of toluene, and the two solutions were mixed to obtain a yellow clear liquid. After reacting at room temperature for 15 hours, the solvent was removed under reduced pressure, concentrated to about 1.0 mL, left to crystallize at room temperature, and 0.60 g of yellow cubic crystals were obtained. The calculated molar yield was 84%.
[0058] Characterization data of catalyst 3 dimethyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com