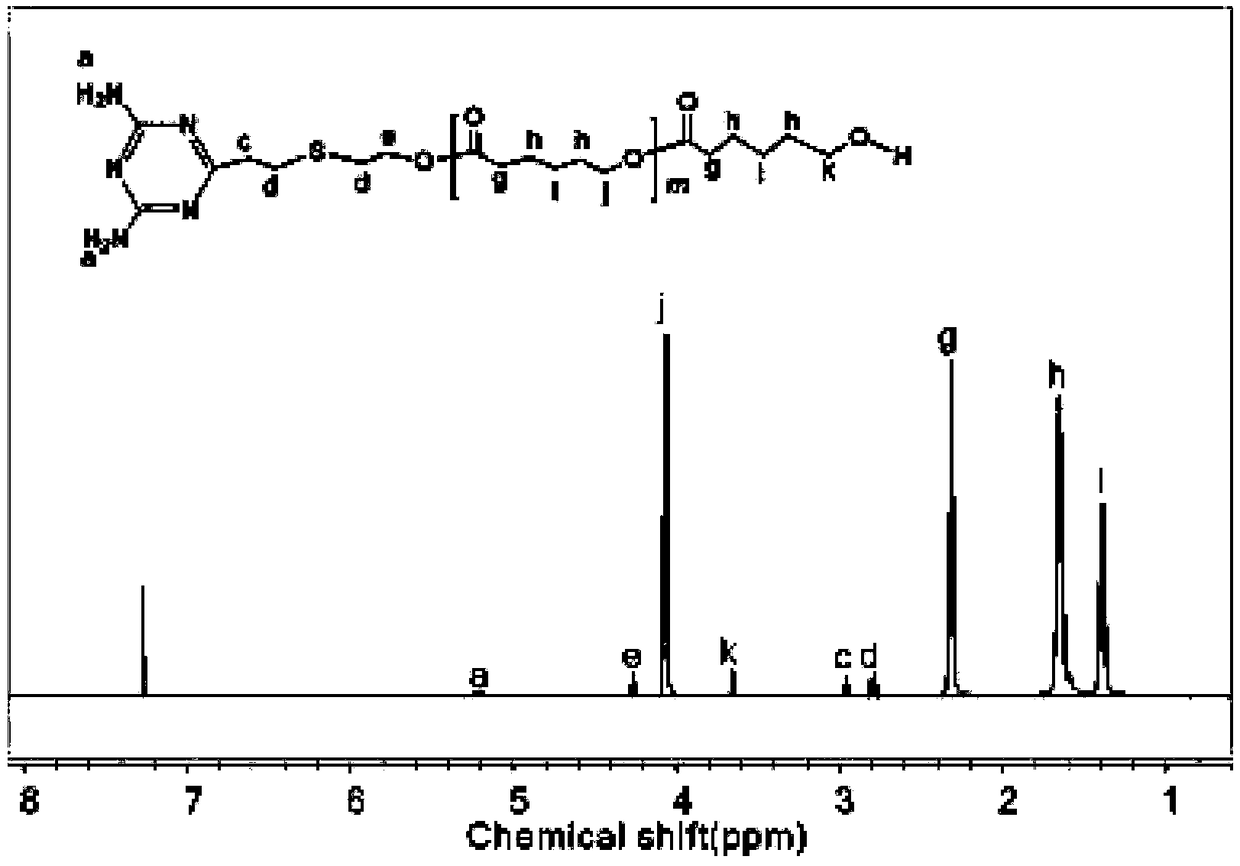
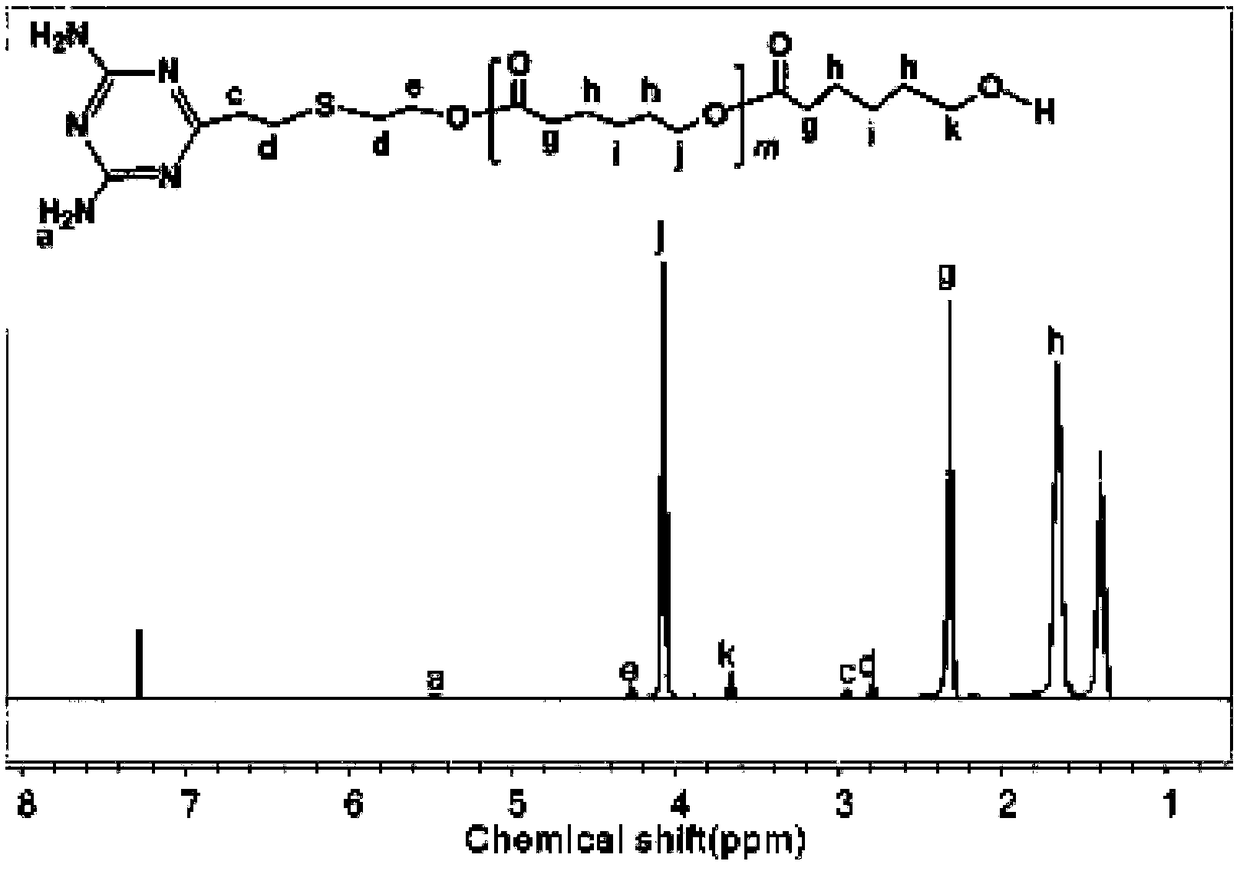
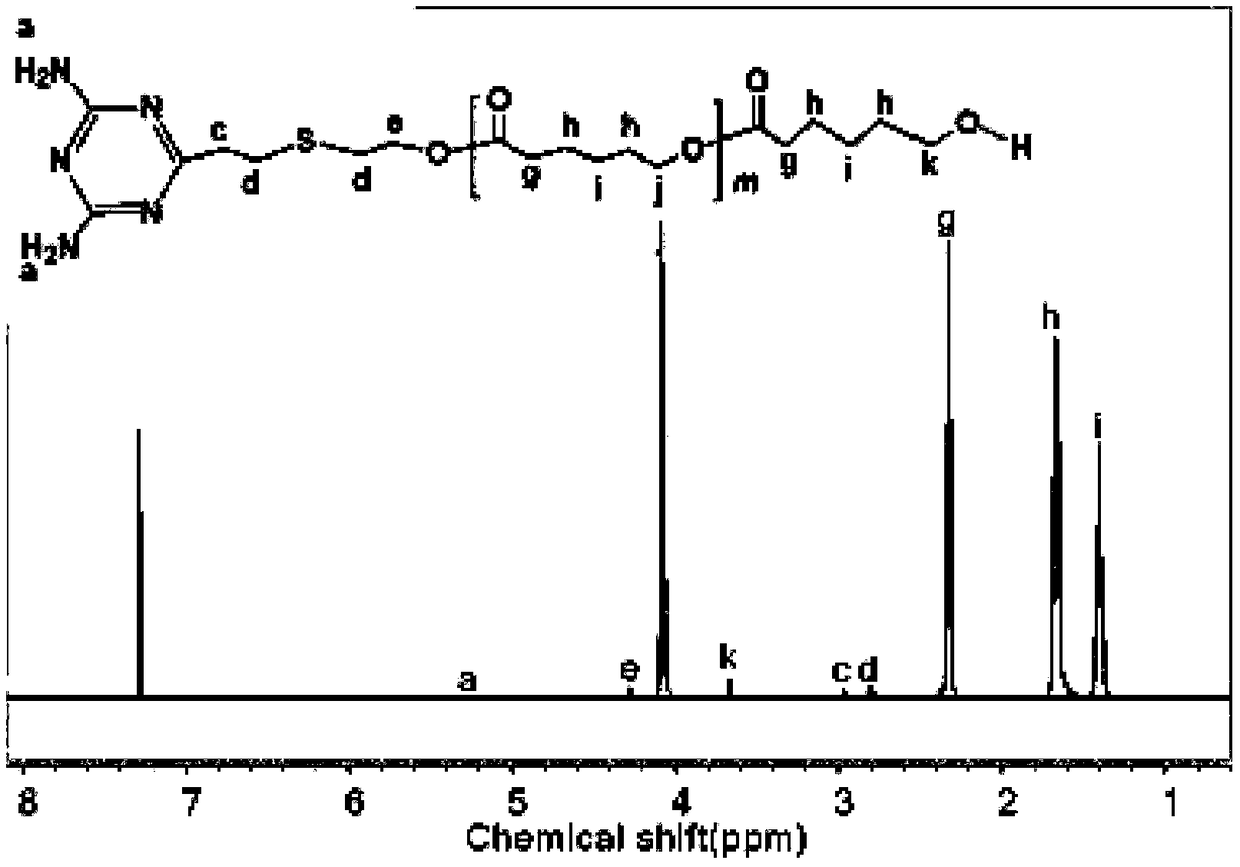
Preparation method of supra-molecular polymer compound micelle based on hydrogen bonds
A technology of supramolecular polymers and composite micelles, which is applied in the field of preparation of supramolecular polymer composite micelles, can solve problems such as strong crystallinity, poor hydrophilicity, and long biodegradation cycle, and achieve mild preparation conditions and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1. Preparation of supramolecular polymer PCL1-DAT...U-PDEA composite micelles based on hydrogen bonds.
[0061] (1) Synthesis of terminal diaminotriazine polycaprolactone (PCL1-DAT) segment
[0062] 1. Synthesis of DAT by click reaction
[0063] Weigh 2.743g (0.02mol) 2,4-diamino-6-vinyl-s-triazine, 0.256g (0.001mol) benzoin dimethyl ether (DMPA), 2.8mL (0.04mol) mercaptoethanol and 70mLN, N-dimethylformamide was placed in a 100mL round bottom flask, stirred at room temperature, and reacted under 365nm ultraviolet light irradiation for 1h after the solid was fully dissolved. After the reaction was completed, it was filtered, and the filtrate was removed by rotary evaporation to obtain a solid. The resulting solid was washed with a mixed solution of 80 mL of acetone and absolute ethanol (1:1), and filtered with suction. Repeat the above operation twice to obtain a white powder. After vacuum drying at 25°C for 24 hours, it is ready for use. The qualitative anal...
Embodiment 2
[0101] Embodiment 2, preparation of supramolecular polymer PCL2-DAT...U-PDEA composite micelles based on hydrogen bond
[0102] The preparation method of the present embodiment 2 is the same as the preparation method of the embodiment 1, and the PCL2-DAT...U-PDEA composite micelle solution with a concentration of 0.5 mg / mL is prepared, the only difference is that the diaminotriazine-terminated polycaprolactone ( The synthetic method of PCL2-DAT) segment, concrete steps are as follows:
[0103] Weigh 0.172g (0.8mmol) DAT, 3.648g (32mmol) caprolactone and 0.007g (0.016mmol) stannous octoate in a 50mL round bottom flask, vacuumize and ventilate nitrogen for six times, under nitrogen protection, 120°C The reaction was stirred for 24h. After the reaction was completed, the reaction mixture was cooled to room temperature. The obtained solid was dissolved in 3 mL of dichloromethane, and the obtained solution was slowly added dropwise to 20 mL (0° C.) ether to precipitate, left to s...
Embodiment 3
[0108] Embodiment 3, preparation of supramolecular polymer PCL3-DAT...U-PDEA composite micelles based on hydrogen bond
[0109] The preparation method of this embodiment 3 is the same as the preparation method of embodiment 1, and the PCL3-DAT...U-PDEA composite micelle solution with a concentration of 0.5 mg / mL is obtained, the only difference is that the diaminotriazine-terminated polycaprolactone ( The synthetic method of PCL3-DAT) segment, concrete steps are as follows:
[0110] Weigh 0.172g (0.8mmol) DAT, 5.472g (48mmol) caprolactone and 0.007g (0.016mmol) stannous octoate in 50mL in a 50mL round-bottomed flask, vacuumize and ventilate nitrogen repeatedly six times, under nitrogen protection, The reaction was stirred at 120°C for 24h. After the reaction was completed, the reaction mixture was cooled to room temperature. The obtained solid was dissolved in 3 mL of dichloromethane, and the obtained solution was slowly added dropwise to 20 mL (0° C.) ether to precipitate, le...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com