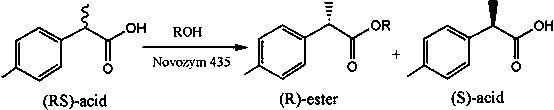
Method for catalytic esterification resolution of 2-(4-methylphenyl) propionic acid enantiomer via stereoselective enzyme
A technology of stereoselectivity and methyl phenyl, which is applied in the field of biocatalytic preparation of chiral compounds, can solve the problems of low thermal stability, low stereoselectivity, and low conversion rate, and achieve improved thermal stability and catalytic efficiency, High reusability, improved purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 2.5 mmol of racemic 2-(4-methylphenyl)propionic acid enantiomer and 2.5 mmol of n-hexanol into a 25 mL volumetric flask, and add n-hexane to make up to volume. In a 25 mL reaction tube, pipette 2 mL of the reaction solution, add 60 mg of different commercial lipases, and heat the reaction at 600 rpm and 50 °C for 5 h; after the reaction, use high performance liquid chromatography The conversion rate of the substrate and the optical purity of the product were analyzed by an instrument. The analysis results showed that when the immobilized Candida antarctica lipase B was used as the catalyst, (R)-2-(4-methylphenyl)propionic acid was preferentially recognized, and the substrate conversion rate was 47.79%. The purity is 27.24%.
Embodiment 2
[0023] In a 25 mL reaction tube, add 0.1 mmol 2-(4-methylphenyl)propionic acid enantiomer and 0.1 mmol n-hexanol as the reaction substrate, 1 mL of different organic solvents as the reaction medium, add 50 mg immobilized Candida antarctica lipase B was reacted at 600 rpm and 50 °C for 1 h. After the reaction, the conversion rate of the substrate and the optical purity of the product were analyzed by high performance liquid chromatography. The analysis results showed that when n-hexane was used as the reaction medium, the conversion rate of the substrate was 53.79 %, and the optical purity of the substrate was 30.91 %.
Embodiment 3
[0025] In a 25 mL reaction tube, add 0.1 mmol 2-(4-methylphenyl)propionic acid enantiomer and 0.1 mmol different kinds of alcohols as reaction substrates, 1 mL n-hexane as reaction medium, add 50 mg immobilized Candida antarctica lipase B was reacted at 600 rpm and 50 °C for 10 min. After the reaction, the conversion rate of the substrate and the optical purity of the product were analyzed by high performance liquid chromatography. The analysis results showed that when n-hexanol was used as the acyl donor, the conversion rate of the substrate was 5.93%, and the optical purity of the substrate was 3.38%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com