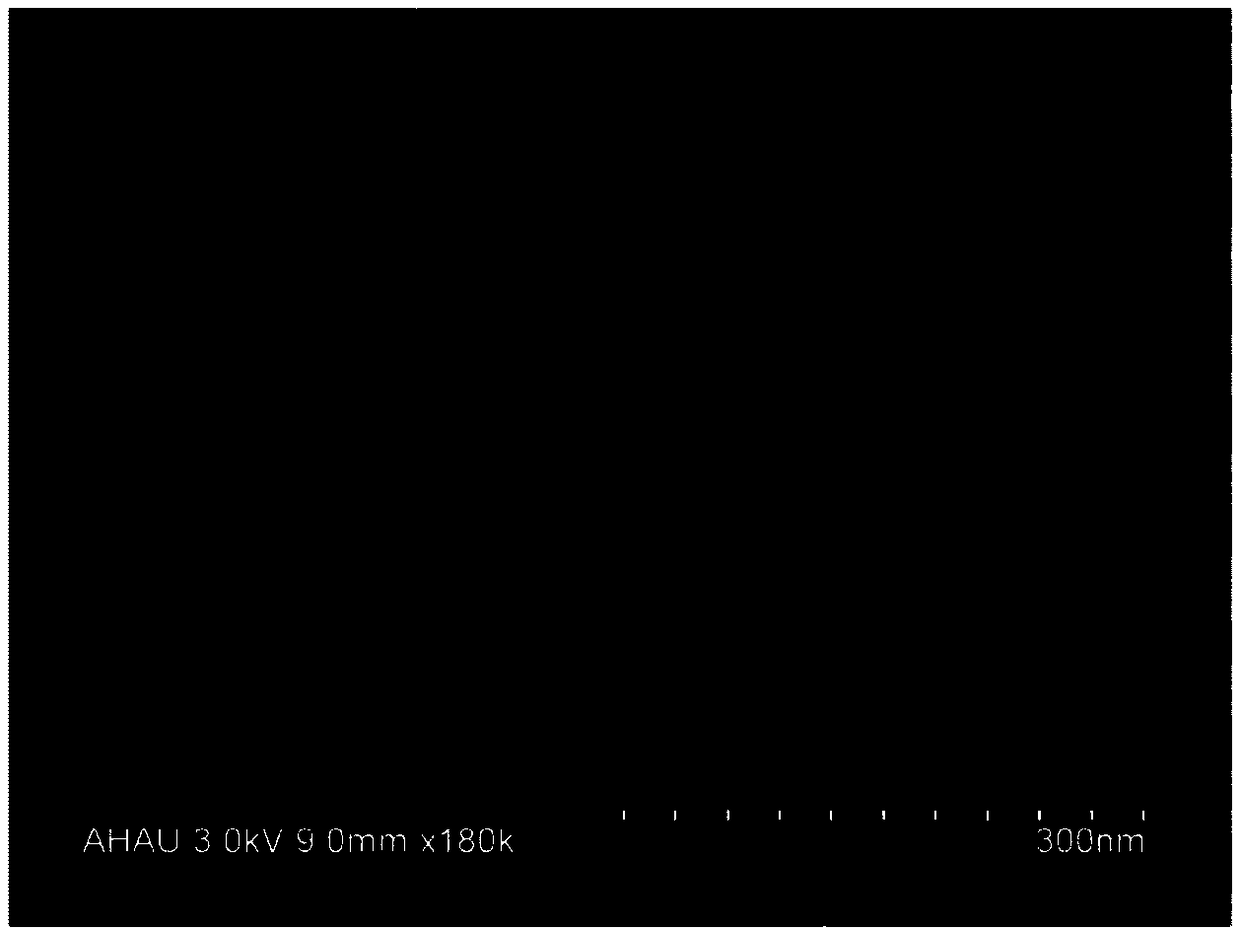
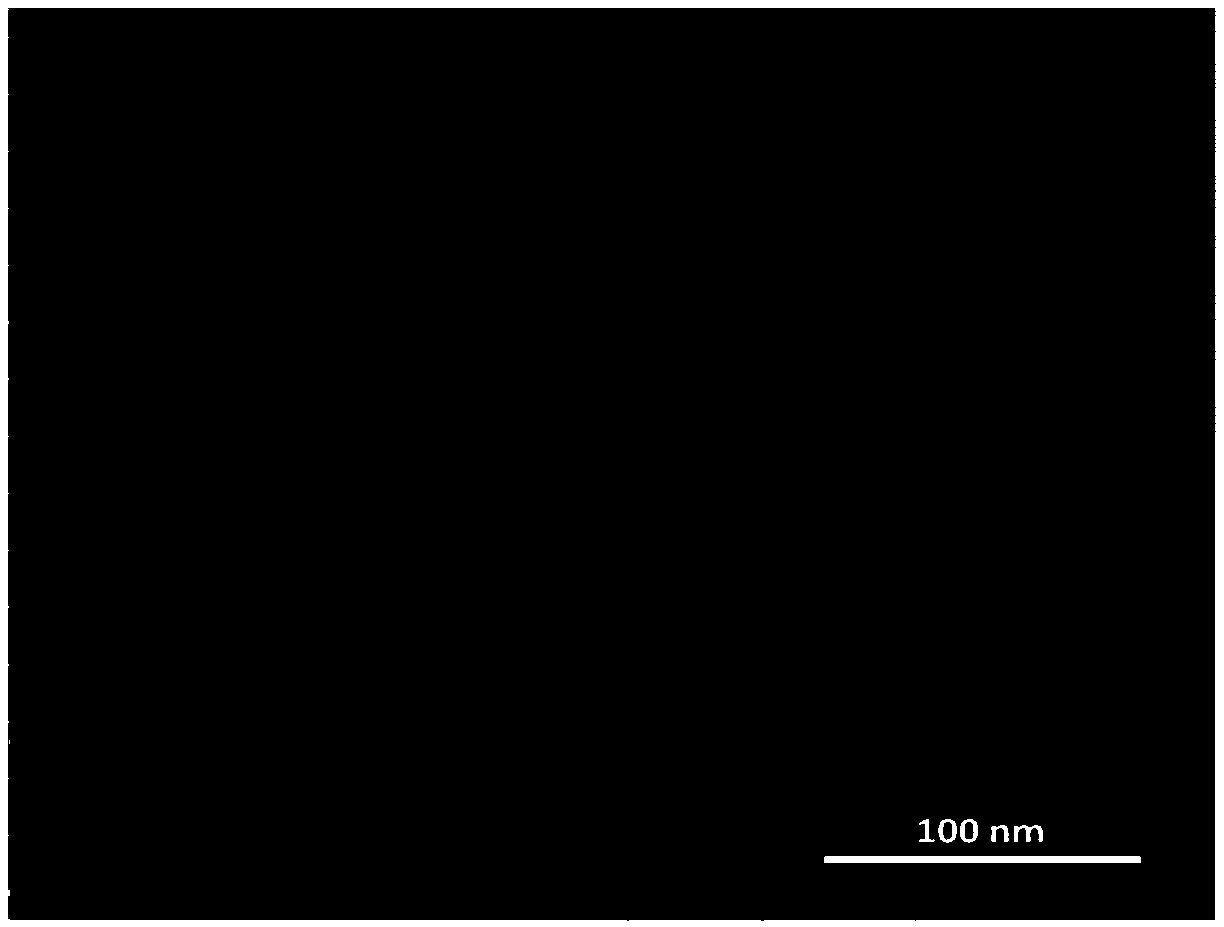
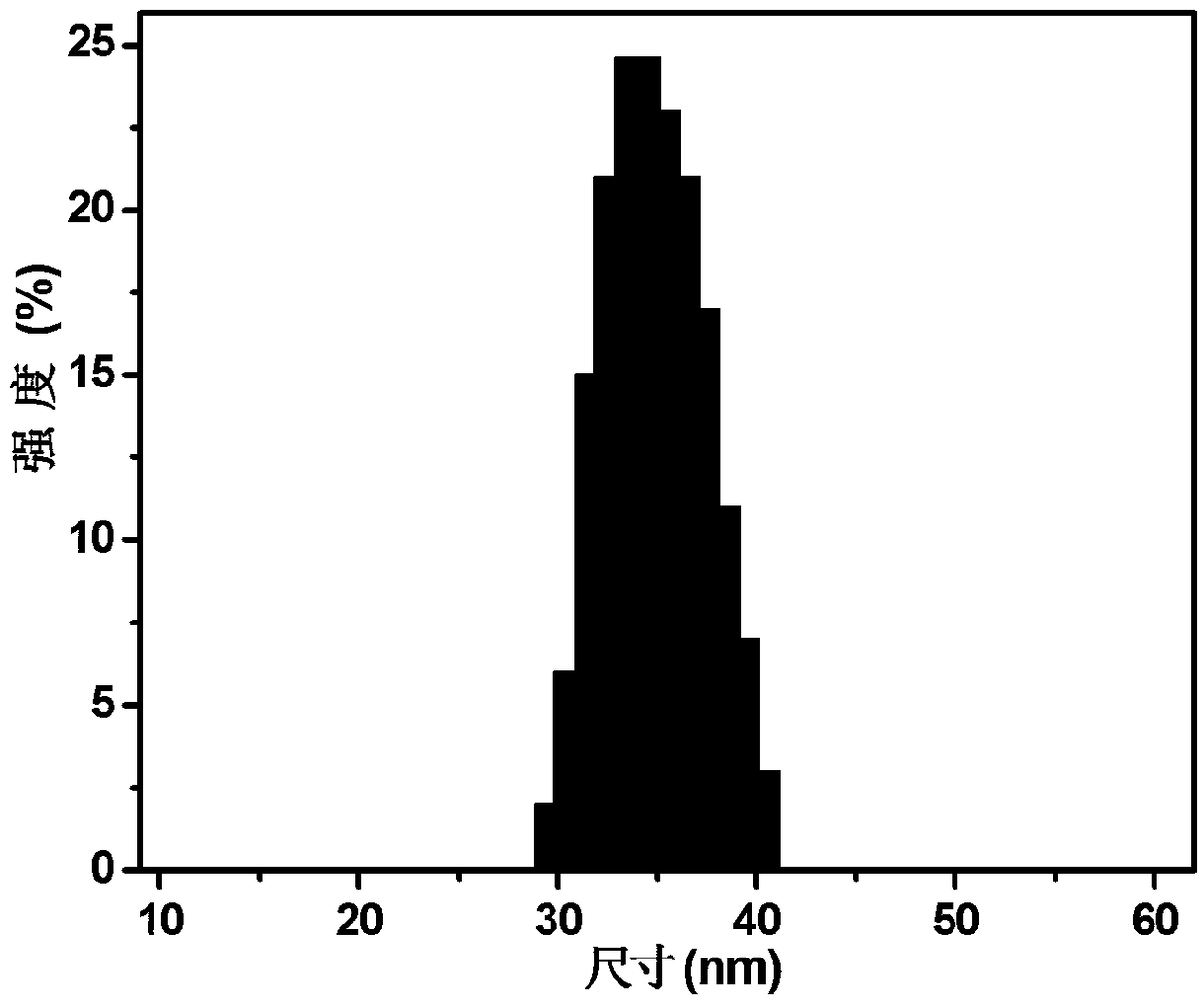
Epigallocatechin-gallate nano slow-release preparation and preparation method thereof
A technology of epigallocatechin and gallate, which is applied in the field of epigallocatechin gallate nano-sustained-release preparations and its preparation, can solve the problems of expensive carrier materials, preparations that cannot be taken orally, and complicated preparation processes, and achieve Wide range of biomedical applications, low requirements for reaction conditions, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of Epigallocatechin Gallate Nanoscale Sustained Release Preparation
[0046] The preparation method comprises the following steps:
[0047] (1) Raw material preparation
[0048] Preparation of oil phase raw materials: uniformly disperse 17 microliters of n-butyl α-cyanoacrylate in 1 milliliter of ethyl acetate;
[0049] Preparation of emulsified phase raw materials: uniformly disperse 25 mg of poloxamer-68 and 50 mg of dextran-70 in 5 ml of ultrapure water, dissolve with ultrasound at room temperature, stir and mix evenly;
[0050] (2) Polymerization reaction
[0051] Accurately weigh 2 mg of epigallocatechin gallate and add it to the oil phase raw material, shake and mix evenly to obtain the mixture I, and then drop the mixture I into the emulsified phase raw material within 6 minutes for interfacial polymerization reaction , the reaction is carried out under magnetic stirring at a rotating speed of 850 rpm, and the total time of the reaction is 3 hours;...
Embodiment 2
[0058] Preparation of Epigallocatechin Gallate Nanoscale Sustained Release Preparation
[0059] The preparation method comprises the following steps:
[0060] (1) Raw material preparation
[0061] Preparation of oil phase raw materials: uniformly disperse 10 microliters of n-butyl α-cyanoacrylate in 1 milliliter of ethyl acetate;
[0062] Preparation of emulsified phase raw materials: uniformly disperse 25 mg of poloxamer-68 and 50 mg of dextran-70 in 5 ml of ultrapure water, dissolve with ultrasound at room temperature, stir and mix evenly;
[0063] (2) Polymerization reaction
[0064] Accurately weigh 1 mg of epigallocatechin gallate and add it to the oil phase raw material, shake and mix evenly to obtain the mixture I, and then drop the mixture I into the emulsified phase raw material within 4 minutes for interfacial polymerization reaction , the reaction is carried out under magnetic stirring at a rotating speed of 800 revolutions per minute, and the total time of the r...
Embodiment 3
[0070] Preparation of Epigallocatechin Gallate Nanoscale Sustained Release Preparation
[0071] The preparation method comprises the following steps:
[0072] (1) Raw material preparation
[0073] Preparation of oil phase raw materials: uniformly disperse 15 microliters of n-butyl α-cyanoacrylate in 1 milliliter of ethyl acetate;
[0074] Preparation of emulsified phase raw materials: uniformly disperse 20 mg of poloxamer-68 and 45 mg of dextran-70 in 5 ml of ultrapure water, dissolve with ultrasound at room temperature, stir and mix evenly;
[0075] (2) Polymerization reaction
[0076] Accurately weigh 2 mg of epigallocatechin gallate and add it to the oil phase raw material, shake and mix evenly to obtain the mixture I, and then drop the mixture I into the emulsified phase raw material within 8 minutes for interfacial polymerization reaction , the reaction is carried out under magnetic stirring at a rotating speed of 900 rpm, and the total time of the reaction is 2.5 hour...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com