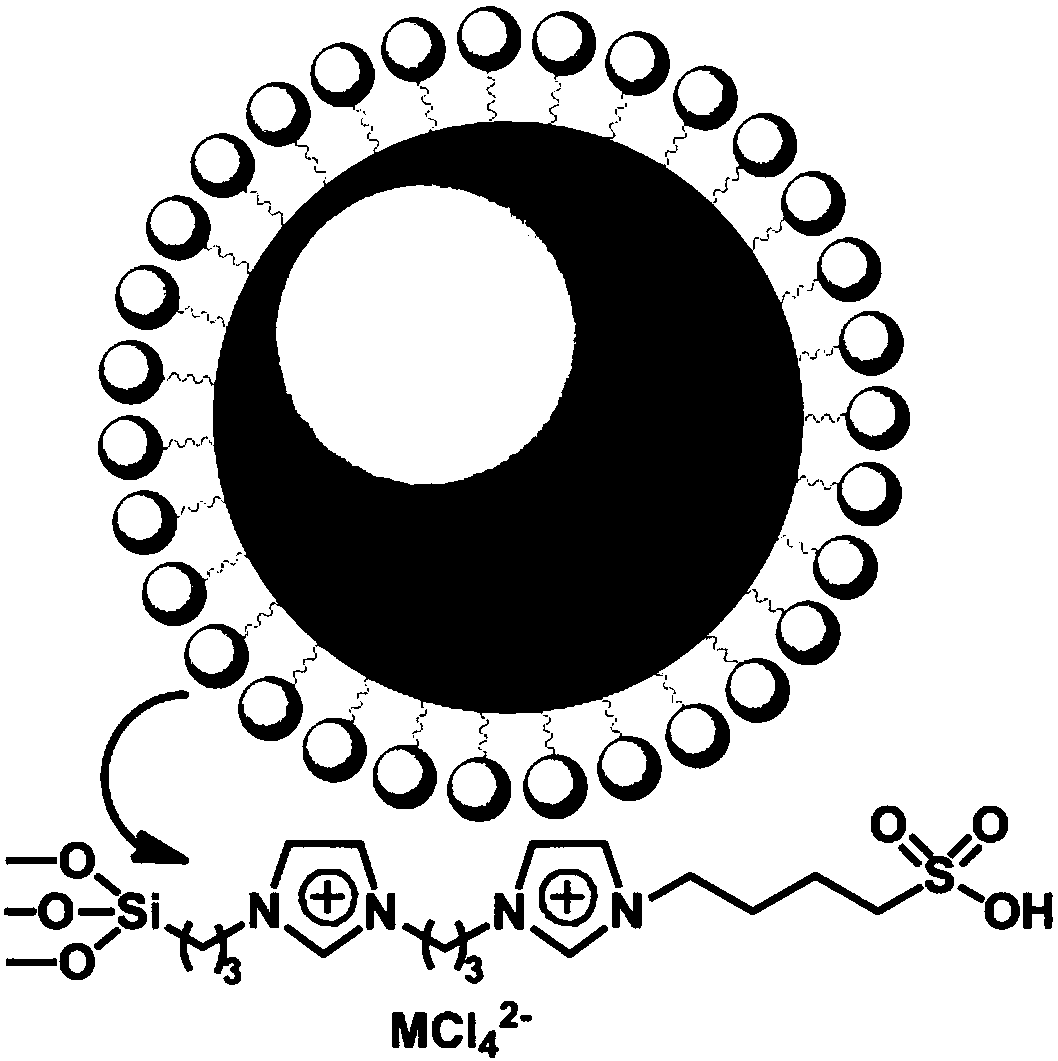
B/L acid-modified organosilicon sphere catalyst, and preparation and application thereof
A catalyst and organosilicon technology, applied in B/L acid-modified organosilicon ball catalyst and its preparation and application field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Catalyst M-S-BIM-SiO 2 preparation
[0016] The preparation of the organosilicon spheres modified by the B / L acid can be operated in the following steps:
[0017] First, add 100mL of methanol, 10mL of ammonia water with a mass fraction of 28% and 30mL of deionized water into a 250mL three-necked flask, stir mechanically for 30 minutes, and then add 3.36g of ethyl orthosilicate and 0.19g of 3-chloro The precursor of propyltriethoxysilane was stirred at 60°C for 3 hours, centrifuged and washed with water to prepare organosilicon spheres for catalysts. Suspend 2 g of the prepared silicone spheres in 30 mL of toluene solution, drop an excess of 0.2 g of diimidazolidine, reflux at 120° C. for 12 hours, centrifuge and wash with water to prepare bis-imidazolidinyl-modified silicone spheres. Suspend 2 g of the prepared bis-imidazolidinyl-modified silicone spheres in 50 mL of toluene solution, add 0.35 g of excess 1,4-butane sultone dropwise into the suspension, stir the mixed...
Embodiment 2
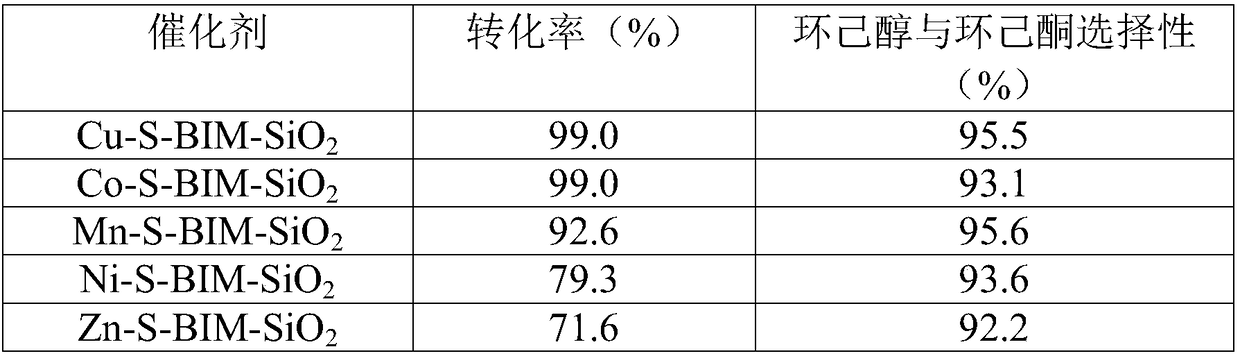
[0019] Catalyst Cu-S-BIM-SiO 2 preparation
[0020] Heterogeneous Catalyst Cu-S-BIM-SiO 2 The preparation method and M-S-BIM-SiO in Example 1 2 The preparation is the same, the difference is that the transition metal chloride salt used is CuCl 2 .
Embodiment 3
[0022] Catalyst Co-S-BIM-SiO 2 preparation
[0023] Heterogeneous Catalyst Co-S-BIM-SiO 2 The preparation method and M-S-BIM-SiO in Example 1 2 The preparation is the same, the difference is that the transition metal chloride salt used is CoCl 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com