Preparing and refining method for hydroxychloroquine and preparation method for sulfate of hydroxychloroquine
A purification method and technology of hydroxychloroquine, applied in the field of preparation and purification of hydroxychloroquine, can solve the problems of unstable impurity control and low purity, and achieve the effects of easy operation, low impurity content and stable impurity content.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
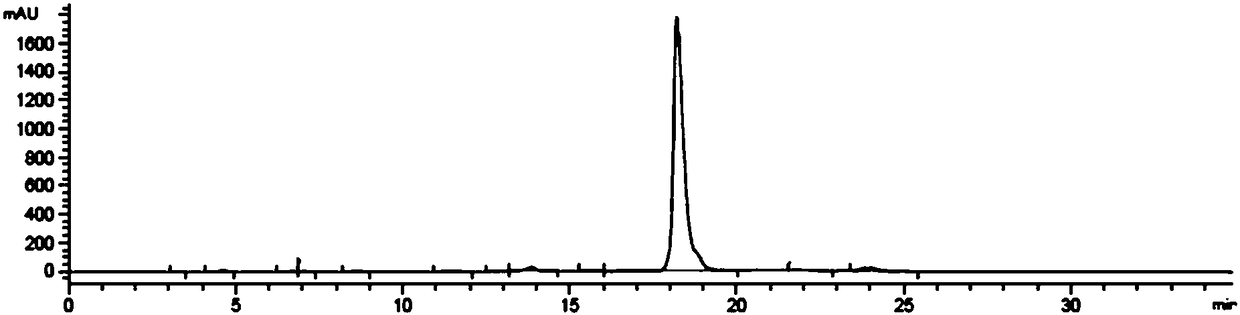
Embodiment 1
[0053] a. Preparation of Hydroxychloroquine
[0054] Add 100g of 4,7-dichloroquinoline and 110g of hydroxychloroquine side chain compound (5-(N-ethyl-N-2-hydroxyethylenediylamino)-2-pentylamine, hereinafter referred to as side chain) into the reactor In the middle, pass nitrogen protection, raise the temperature to 78°C to dissolve 4,7-dichloroquinoline, raise the temperature to 120°C for 20 minutes, raise the temperature to 130°C for 8 hours, cool down (below 80°C) after the reaction, and use sodium hydroxide solution (mass concentration is 7%) to adjust pH>12, extract with dichloromethane, wash with water until neutral, evaporate dichloromethane under reduced pressure to obtain 154 g of crude hydroxychloroquine, yield 90.7%, HPLC purity 92.45%. Add 300g methyl ethyl ketone and 300g ethyl acetate to the full amount of crude hydroxychloroquine, heat at 75°C to dissolve, slowly cool down to 10°C over 4 hours, filter, and wash the filter cake with a mixed solution of methyl ethy...
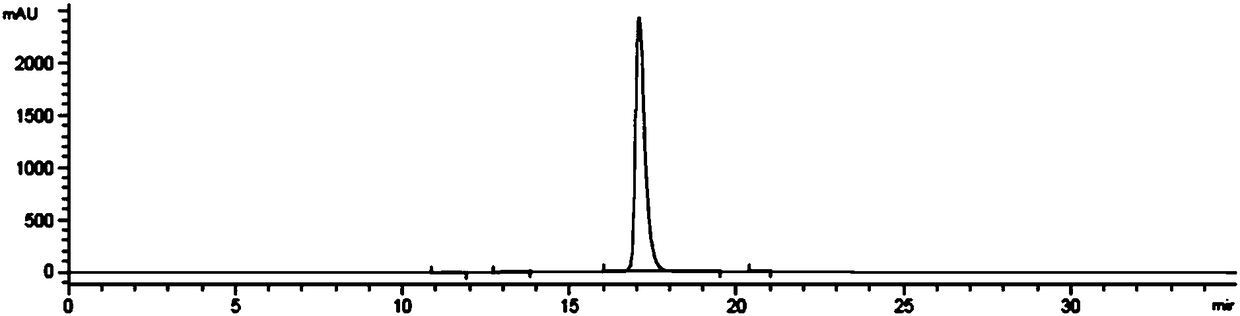
Embodiment 2
[0057] Embodiment 2: the preparation of hydroxychloroquine sulfate
[0058] a. Preparation of Hydroxychloroquine
[0059] Add 100g of 4,7-dichloroquinoline and 130g of side chains into the reactor, pass through argon protection, raise the temperature to 70°C to dissolve the 4,7-dichloroquinoline, raise the temperature to 115°C for 10 minutes, and raise the temperature to 137°C to react After 10 hours, the temperature was lowered (below 80°C) after the reaction was completed, the pH was adjusted to >12 with sodium hydroxide solution (6% mass concentration), extracted with dichloromethane, washed with water until neutral, dichloromethane was distilled off under reduced pressure to obtain hydroxychloroquine The crude product was 157g, the yield was 92.5%, and the HPLC purity was 93.96%. Add 200 g of acetone and 250 g of methyl acetate to the crude product of hydroxychloroquine, heat to dissolve at 65°C, slowly cool down to 10°C over 4 hours, filter, and wash the filter cake with...
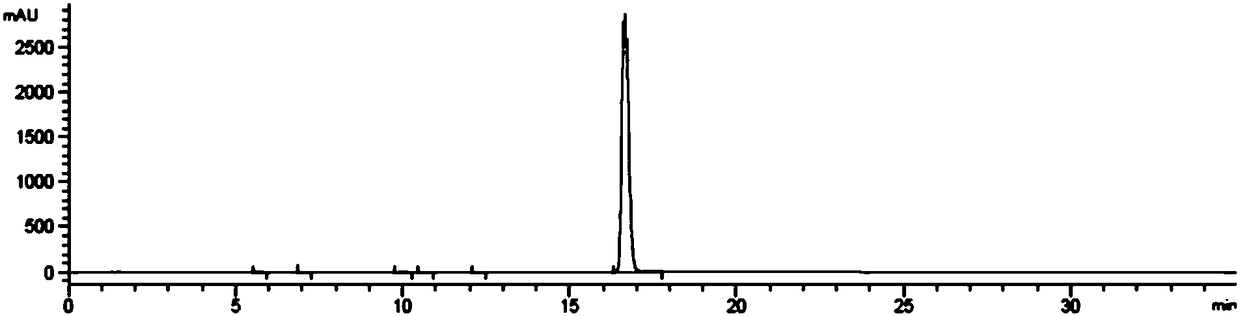
Embodiment 3
[0062] Embodiment 3: the preparation of hydroxychloroquine sulfate
[0063] a. Preparation of Hydroxychloroquine
[0064] Add 100g of 4,7-dichloroquinoline and 130g of side chains into the reactor, pass through helium protection, raise the temperature to 70°C to dissolve the 4,7-dichloroquinoline, raise the temperature to 115°C for 15 minutes, and raise the temperature to 135°C to react After 11 hours, the temperature was lowered (below 80°C) after the reaction was completed, and the pH was adjusted to >12 with sodium hydroxide solution (mass concentration: 10%), extracted with dichloromethane, washed with water until neutral, and dichloromethane was evaporated under reduced pressure to obtain hydroxychloroquine The crude product was 158g, the yield was 93.1%, and the HPLC purity was 93.73%. Add 400g of 2-pentanone and 350g of isopropyl acetate to the crude product of hydroxychloroquine, heat to dissolve at 65°C, slowly cool down to 10°C over 4 hours, filter, and use a mixed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com