A kind of preparation method of pentazocine intermediate
A technology of pentazocine and its intermediates, which is applied in the field of chemical synthesis and can solve the problems of resource waste and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] The preparation method of the pentazocine intermediate provided in this embodiment, the prepared pentazocine intermediate is shown in formula (1):
[0068]
[0069] The reaction equation of its preparation process is as shown in formula (2):
[0070]
[0071] Its preparation process comprises the following steps:
[0072] Step 1: Methylation reaction
[0073] Add potassium carbonate and methyl iodide to compound 1 in acetone medium, and react at 50°C to obtain compound 2. The molar ratio of compound 1 to methyl iodide is 1:1.3-1.5, and methyl iodide can also be dimethyl sulfate substitute;
[0074] Step 2: Ring opening reaction
[0075] Add concentrated hydrochloric acid and sodium chloride solution to compound 2, and react at 50°C to obtain compound 3, the molar ratio of compound 2 to hydrochloric acid is 1:2.0-2.5, wherein, concentrated hydrochloric acid can be one of phosphoric acid, sulfuric acid and nitric acid an alternative;
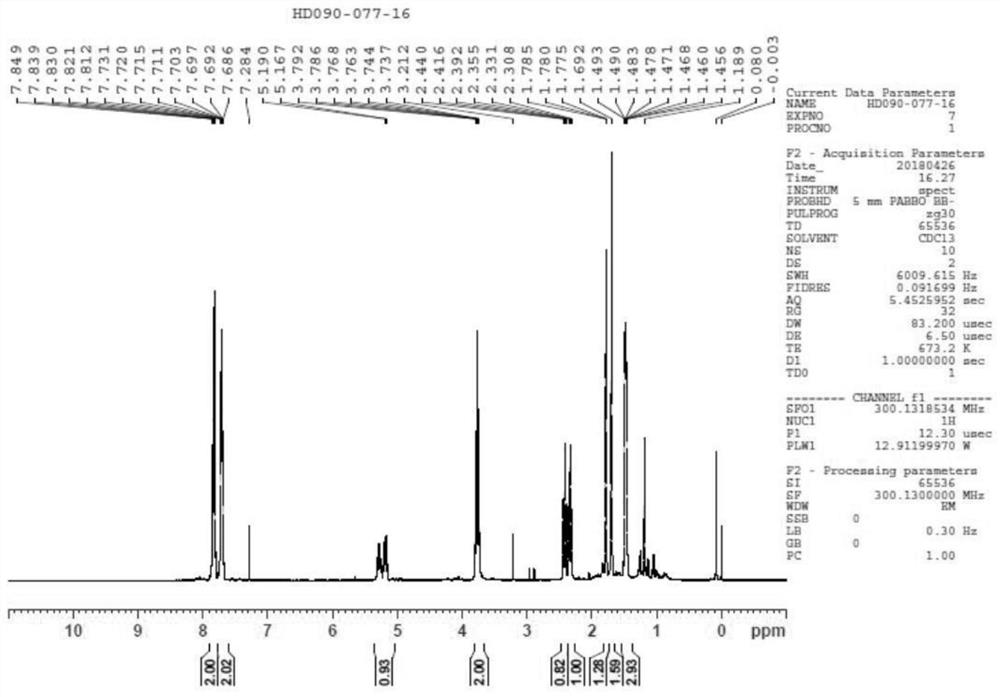
[0076] Step 3: Reduction ...
Embodiment 1
[0096]
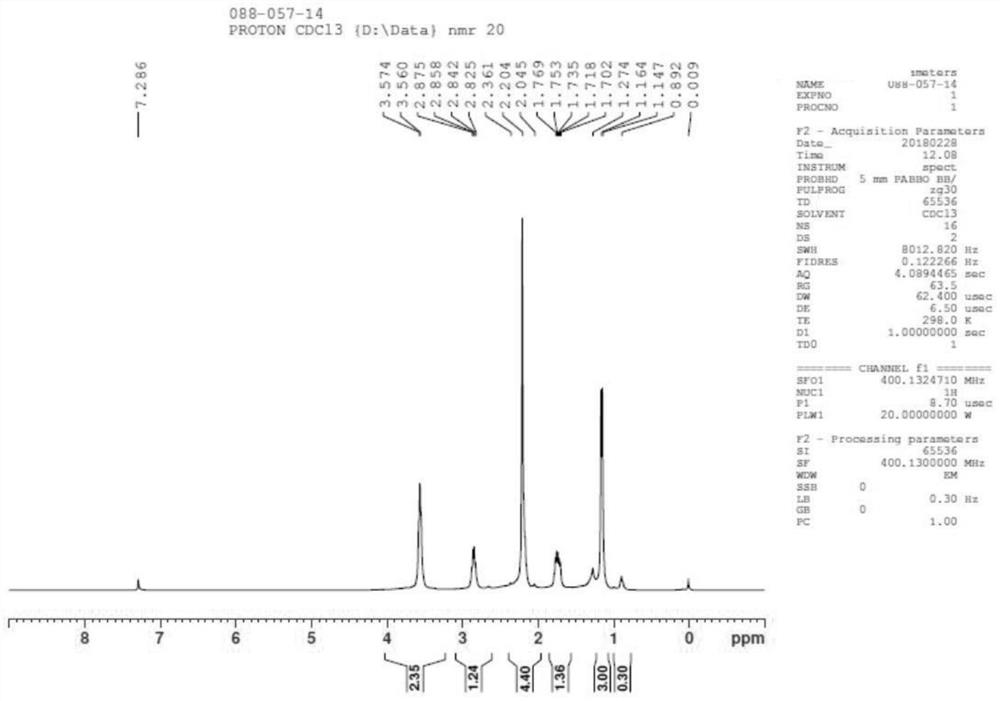
[0097] Step 1: Add compound 1 (200g), acetone (2L) to a 3L four-neck flask, stir, add potassium carbonate (280.1g, 1.3eq), stir, then add methyl iodide (288.2g, 1.3eq), and heat up To 50°C (micro-reflux), keep warm for 16h, GC sampling detection: raw material <2%; Post-treatment: filter, wash the solid twice with a small amount of acetone (200ml), spin dry the acetone, add water (800ml) + ethyl acetate ( 800ml), stirred, left to stand for layering, the aqueous phase was extracted once with ethyl acetate (800ml), the organic phases were combined, the organic layer was washed once with saturated NaCl (600ml), dried over anhydrous sodium sulfate, and spin-dried to obtain 199g of liquid compound 2; Yield: 90%;
[0098]
[0099] Step 2: Add concentrated hydrochloric acid (286ml, 2.0eq) to compound 2 (200g, 1.0eq), then add 200ml of 15% NaCl solution, heat to 50 degrees to react, take a sample for about 4 hours, send the sample to GC, post-processing: direct distillat...
Embodiment 2
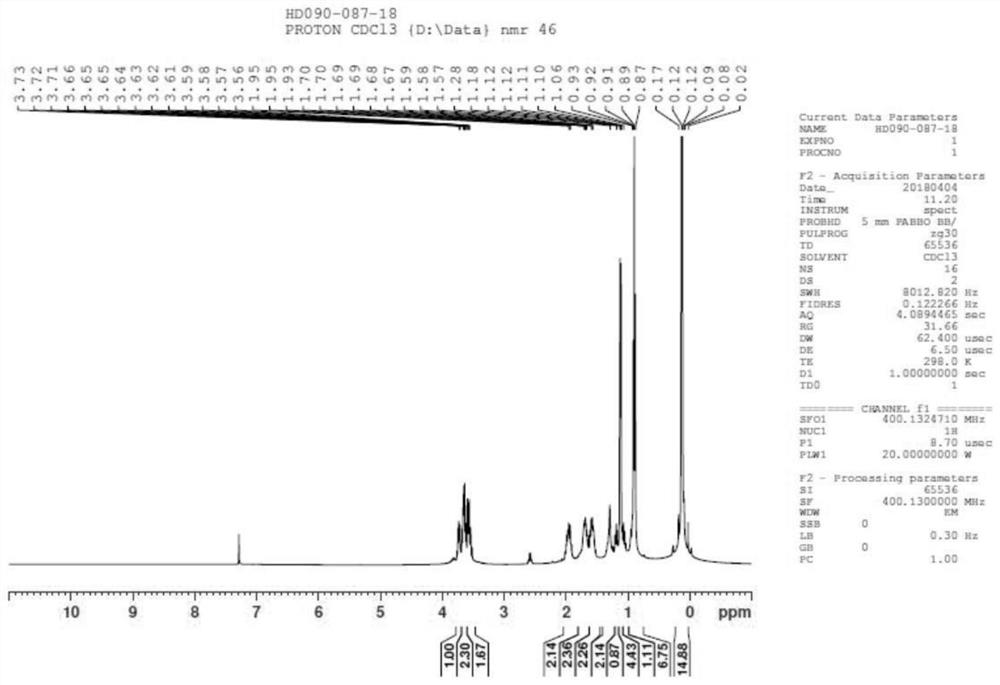
[0117] Step 1: Add compound 1 (2000g), acetone (20L) to a four-neck flask, stir, add potassium carbonate (2801g, 1.3eq), stir, then add methyl iodide (2883g, 1.3eq), and heat up to 50°C (Slight reflux), keep warm for 16h, GC sampling detection,; post-treatment: filter, wash the solid twice with a small amount of acetone (2000ml), spin dry the acetone, add water (8000ml)+ethyl acetate (8000ml), stir, let stand The layers were separated, the aqueous phase was extracted once with ethyl acetate (8000ml), the organic phases were combined, the organic layer was washed once with saturated NaCl (6000ml), dried over anhydrous sodium sulfate, and spin-dried to give 1997g of liquid compound 2; yield: 95% ;
[0118] Step 2: Compound 2 (400g, 1.0eq), add concentrated hydrochloric acid (500ml, 2.0eq), then add 400ml15% NaCl solution, heat to 50 degrees to react, take a sample for about 4h, send the sample to GC, post-processing: direct distillation reaction liquid, distilled water at norma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com