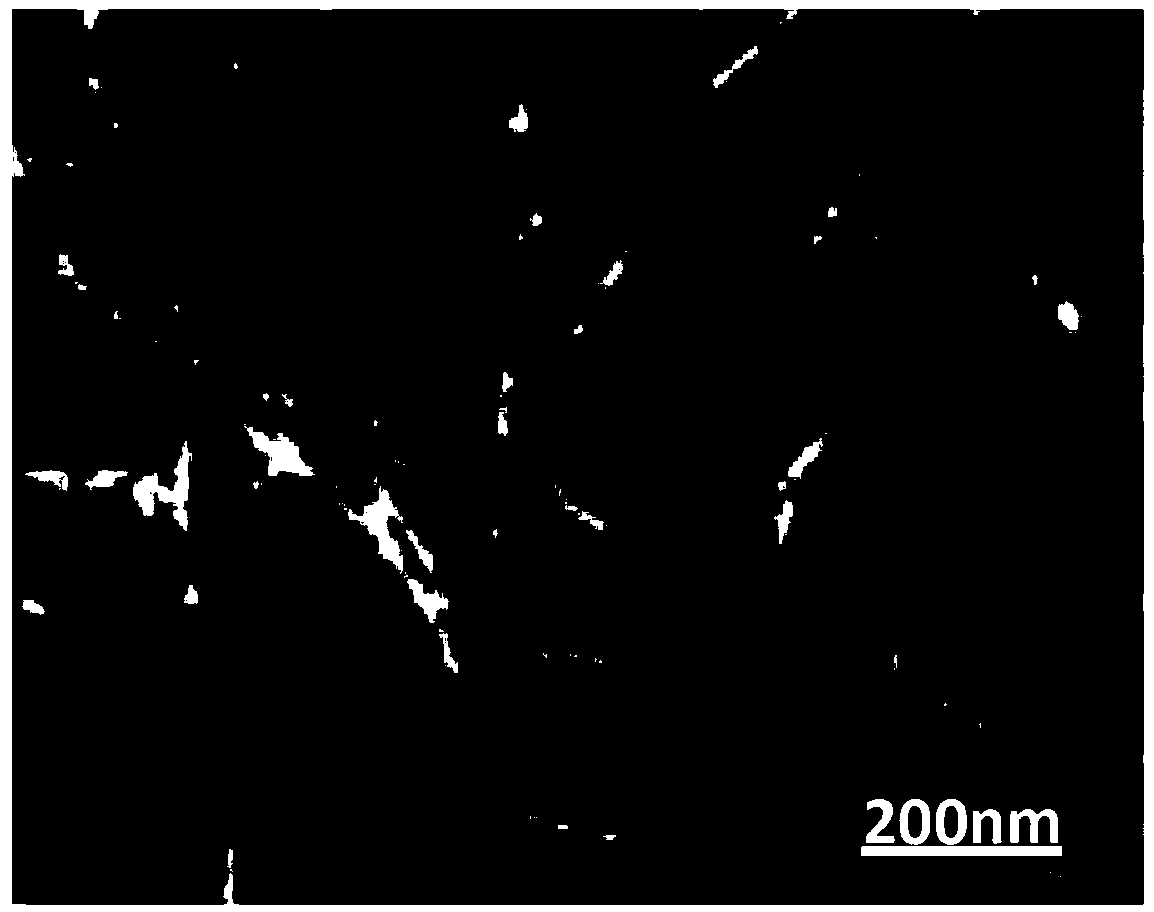
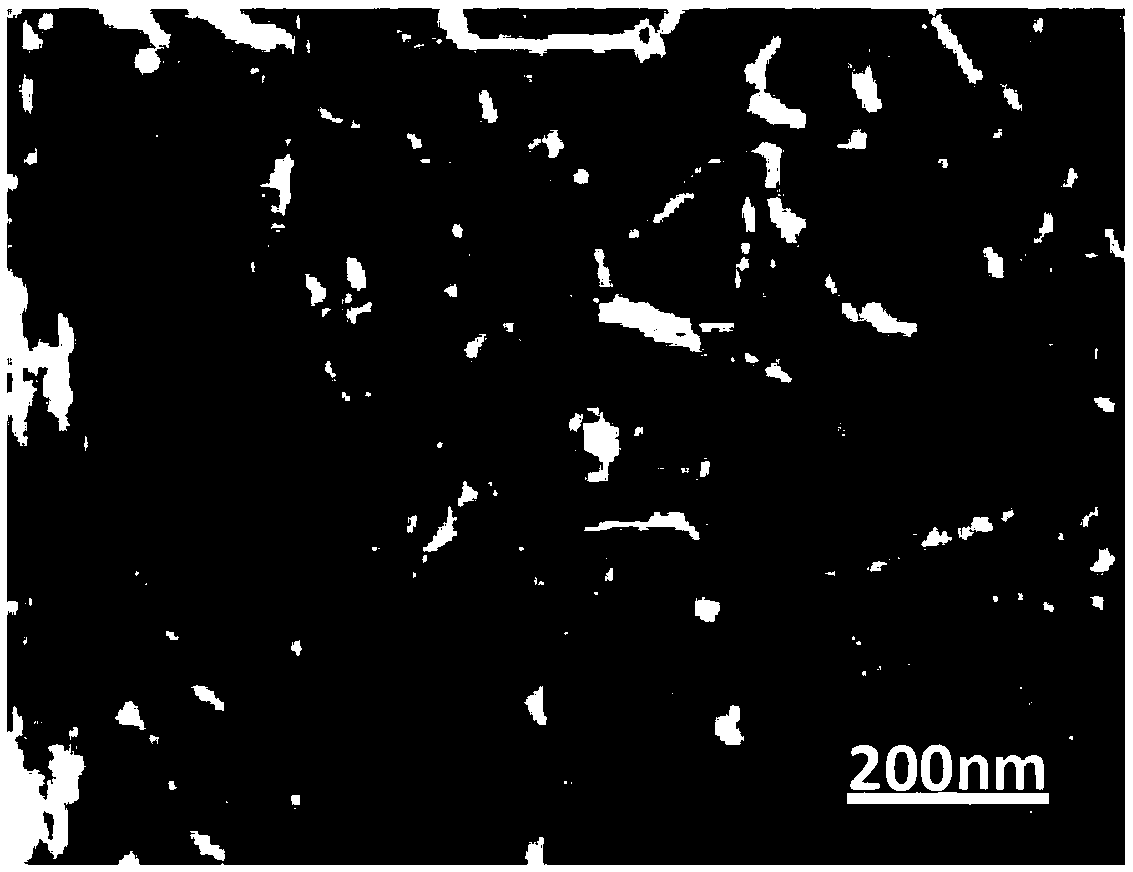
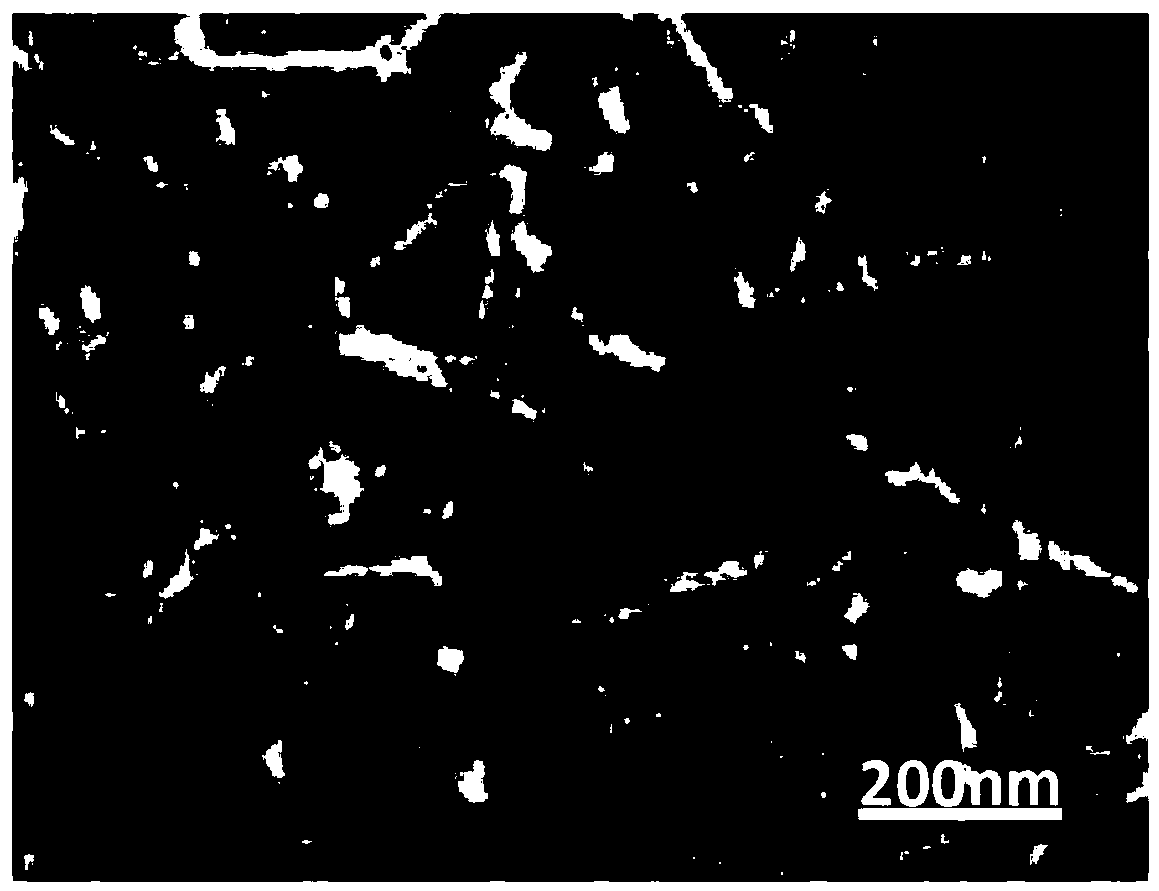
Preparation methods of immobilized nitrile group hydratase and (S)-N-ethylpyrrolidine-2-formamide
A technology for hydration of ethylpyrrolidine and nitrile group, applied in the field of biomedicine, can solve the problems of expensive chiral raw materials and chiral catalysts, low atom utilization rate of chemical resolution method, asymmetric chiral catalysts, etc. The effect of low cost, low production cost and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Preparation of carboxylated carbon nanotubes:
[0053] In a dry and clean reactor, add 30g carbon nanotubes (purity 95%, Chengdu Organic Chemistry Co., Ltd., Chinese Academy of Sciences) and 60mL mixed acid (mass fraction 98% concentrated sulfuric acid: mass fraction 68% concentrated nitric acid volume ratio is 3:1, The volume dosage is 2mL / g based on the mass of carbon nanotubes) After ultrasonic treatment for 1 hour, heat and reflux for 12 hours to react, filter, wash with distilled water until the pH is 7.0, and dry under reduced pressure and vacuum at 50°C to obtain carboxylated carbon nanotubes. Tube.
Embodiment 2
[0055] Preparation of cyclodextrin-carbon nanotube-immobilized nitrile hydratase (abbreviated as E1):
[0056] (1) Feeding amount: carboxylated carbon nanotube 5g; N,N-dimethylformamide is an organic base, and the feeding amount is 0.25g, and its quality consumption is 5% g / g of the quality of carboxylated carbon nanotube; Chlorine Sulfoxide 25g, its quality dosage is 5 times g / g of carboxylated carbon nanotube quality; Cyclodextrin (98%, Shanghai Aladdin Biochemical Technology Co., Ltd., viscosity 200-400mPa.s) is polysaccharide, and feed amount 15g, its mass dosage is 3 times g / g of carboxylated carbon nanotube quality; Triethylamine is an organic amine, the feeding amount is 10g, its mass dosage is 2 times of carboxylated carbon nanotube quality g / g; N,N -Dimethylformamide is the organic solvent A, the feeding amount is 150mL, and its volume consumption is 30mL / g in terms of carboxylated carbon nanotube quality;
[0057] In a dry and clean reactor, add thionyl chloride, ca...
Embodiment 3
[0061] Preparation of chitosan-carbon nanotube-immobilized nitrile hydratase (abbreviated as E2):
[0062] (1) Feeding amount: carboxylated carbon nanotube 5g; N,N-dimethylacetamide is an organic base, and the feeding amount is 0.5g, and its quality consumption is 10% g / g of the quality of carboxylated carbon nanotube; Chlorine Sulfoxide 40g, its quality consumption is 8 times g / g of carboxylated carbon nanotube quality; The quality dosage is 5 times g / g of the quality of carboxylated carbon nanotubes; dimethylaminopyridine is an organic amine, and the feeding amount is 15g, and its quality dosage is 3 times g / g of the quality of carboxylated carbon nanotubes; DMSO is organic solvent A , the feeding amount is 150mL, and the volumetric dosage is 30mL / g based on the mass of carboxylated carbon nanotubes;
[0063] The reaction temperature was 100° C., the reaction time was 48 hours, and other operations were the same as in Example 2 to obtain a chitosan-carbon nanotube composite...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com