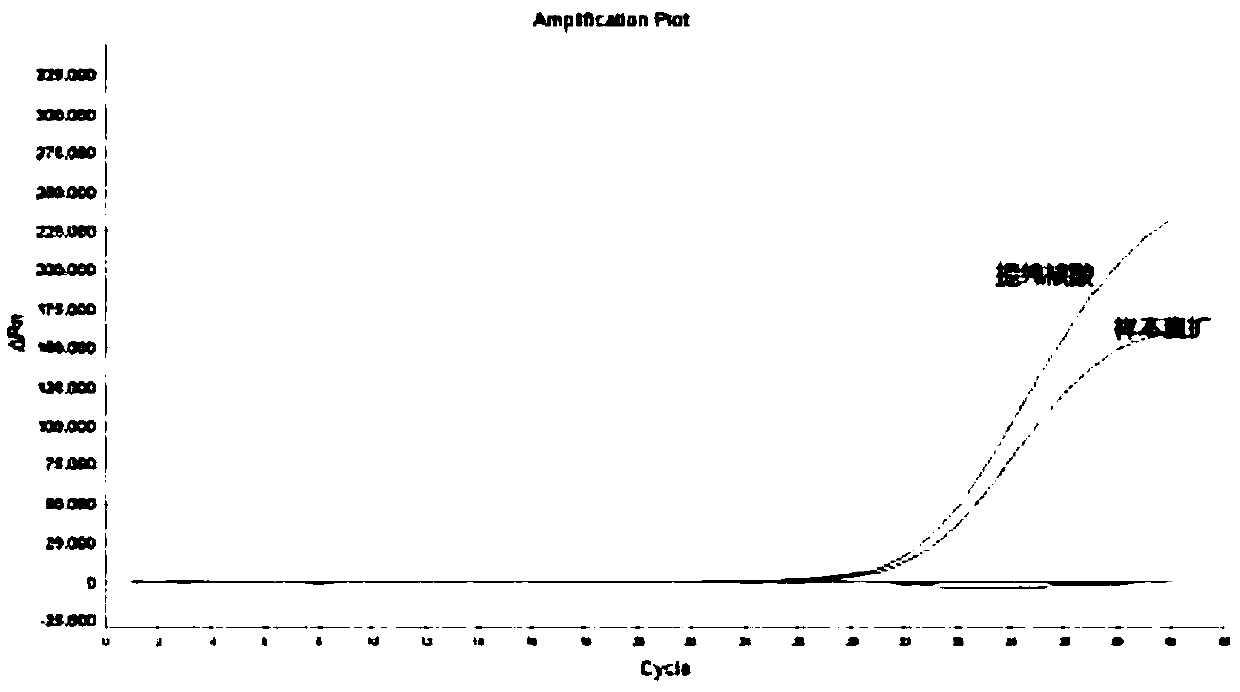
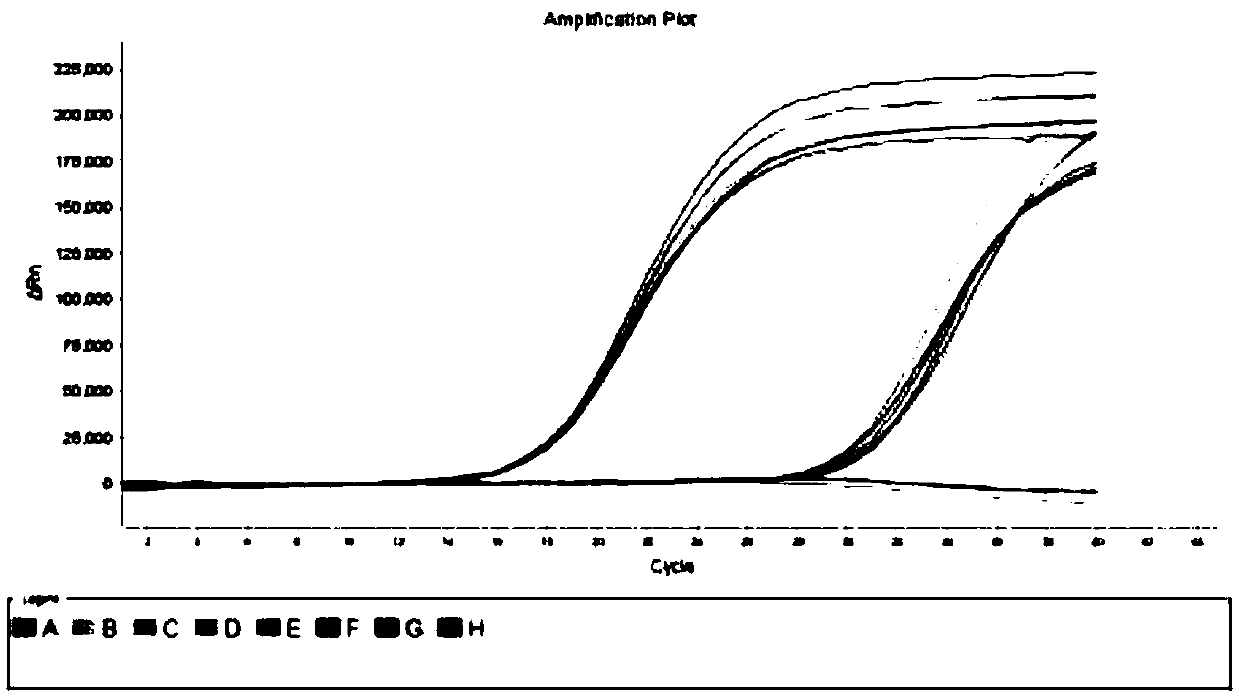
A detection kit for human parainfluenza virus nucleic acid extraction-free gene typing
A human parainfluenza virus and detection kit technology, applied in the fields of biotechnology and medicine, can solve the problems of long time-consuming and low detection efficiency of single-plex detection, and achieve the effects of good accuracy, high precision and reliability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] 1. Sample preparation in the sample processing area
[0134] 1.1. This kit does not require nucleic acid extraction, and can directly amplify and detect viral nucleic acid in throat swab samples and serum samples. Before the experiment, the throat swab samples only need to be thawed and vortexed, and blood samples need to be centrifuged to obtain serum;
[0135] 2. Prepare PCR reagents in the reagent preparation area
[0136] 2.1. Prepare the PCR reaction solution according to the following composition (n is the number of reaction tubes):
[0137] HPIV PCR reaction buffer 1 is 19 μl×n, HPIV1 / 2 / 3 / 4 probe mixture is 2 μl×n, enzyme system 3 is 2 μl×n, and the PCR reaction buffer and primer-probe mixture are fully prepared before use. To dissolve, the enzyme system needs to be centrifuged before use to ensure that all enzymes are concentrated at the bottom;
[0138] 2.2. Dispense the PCR reaction solution into PCR reaction tubes according to 23 μl / tube, and move the react...
Embodiment 2
[0157] Three batches of human parainfluenza virus RNA detection kits were used to carry out 10 repeated detections of the positive control, negative control, medium and low concentrations of self-made standard products, and the CV range of the imprecision Ct value within the batch was : HPIV-1 is 0.68~1.42%, HPIV-2 is 0.72~1.55%, HPIV-3 is 0.77~1.47%, HPIV-4 is 0.89~1.65%; the CV range of the imprecision Ct value between batches is: HPIV -1 is 1.12-1.19%, HPIV-2 is 1.04-1.40%, HPIV-3 is 1.03-1.52%, HPIV-4 is 0.95-1.40%; the CV values of intra-assay and inter-assay precision Ct values are less than 5 %, the negative controls were all negative, indicating that the kit has good precision, and the test results are as follows in Table 1:
[0158] Table 1
[0159]
Embodiment 3
[0161] Select other pathogens that have homology to the nucleic acid sequence of human parainfluenza virus, are likely to cause the same or similar clinical symptoms, and are normally parasitized or easily complicated at the sampling site, such as influenza A virus, influenza B virus, influenza C virus, nasal Viruses, respiratory syncytial virus, adenovirus, measles virus, rubella virus, and mumps virus are the samples to be tested, and the qualified human parainfluenza virus nucleic acid typing detection kit is used for detection, and the operation is strictly in accordance with the kit instructions The test was carried out on an ABI7500 real-time fluorescent quantitative PCR instrument, and the test results were all negative, indicating that the specificity of the kit was good.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com