Licorice root residue fermentation health-care feed and production technology thereof
A production process, the technology of licorice residue, which is applied in the field of licorice residue fermented health feed and its production technology, can solve the problems of threatening human life and health, increasing human drug resistance, wasting resources, etc., to increase the additional application value and improve the quality of animal products. Quality, the effect of promoting animal growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
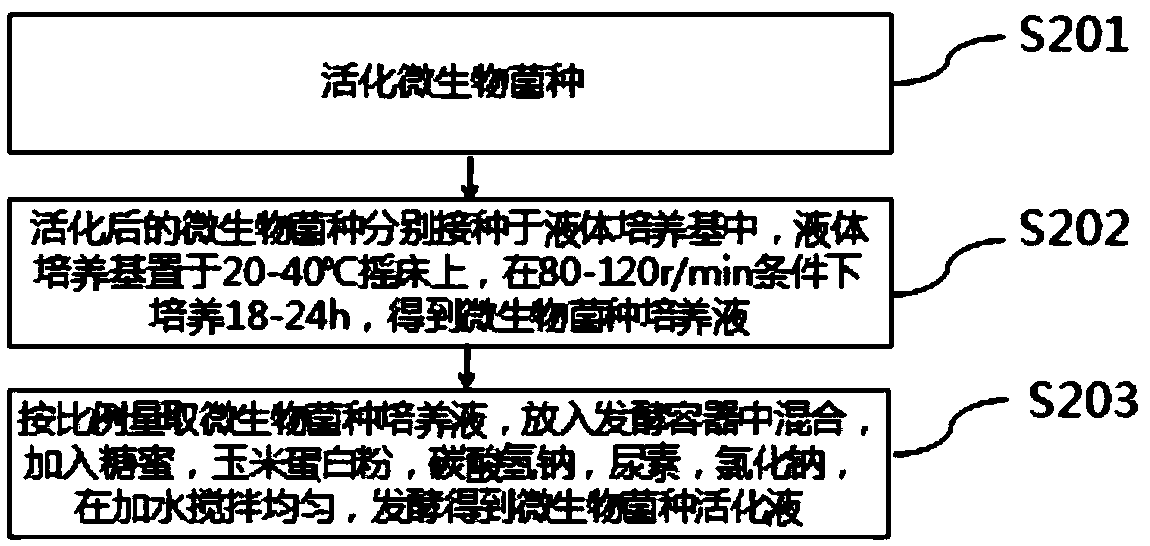
[0072] Preparation of microbial strain activation solution:
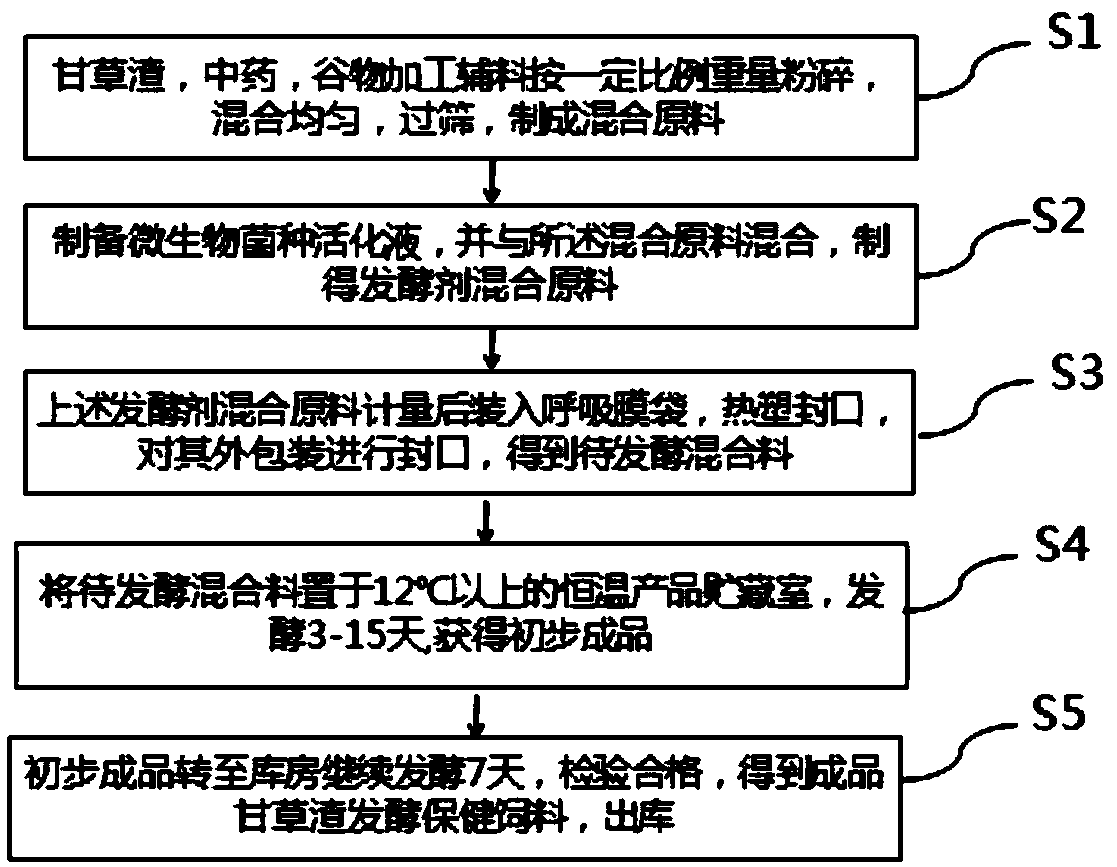
[0073] Specifically, Bacillus subtilis, Saccharomyces cerevisiae and Lactobacillus acidophilus were respectively activated on the solid medium, and the above-mentioned activated microbial strains were respectively inoculated in the liquid medium, and the liquid medium was placed on a shaker at 30° C. Cultivate under the condition of 120r / min for 24 hours to obtain the culture medium of the cultured microorganisms; weigh 06L of the culture medium of Bacillus subtilis, 0.3L of the culture medium of Saccharomyces cerevisiae and 0.1L of the culture medium of Lactobacillus acidophilus, put them into the fermentation container, add molasses 2kg, corn gluten powder 200g, sodium bicarbonate 50g, urea 30g, sodium chloride 50g, add water 100L, stir evenly, ferment to obtain microbial strain activation liquid.
[0074] Weigh the following raw materials: licorice residue 100kg, traditional Chinese medicine 20kg, grain processin...
Embodiment 2
[0081] Specifically, Bacillus subtilis, Saccharomyces cerevisiae and Lactobacillus acidophilus were respectively activated on the solid medium, and the above-mentioned activated microbial strains were respectively inoculated in the liquid medium, and the liquid medium was placed on a shaker at 30° C. Cultivate under the condition of 120r / min for 24 hours to obtain the culture medium of the cultured microorganisms; weigh 06L of the culture medium of Bacillus subtilis, 0.3L of the culture medium of Saccharomyces cerevisiae and 0.1L of the culture medium of Lactobacillus acidophilus, put them into the fermentation container, add Molasses 2kg, corn gluten powder 200g, sodium bicarbonate 50g, urea 30g, sodium chloride 50g, add water 130L, stir evenly, ferment to obtain microbial strain activation liquid.
[0082] Weigh the following raw materials: licorice residue 150kg, traditional Chinese medicine 30kg, grain processing auxiliary materials (flax cake, soybean meal, DDGS, corn, ric...
Embodiment 3
[0089] Specifically, Bacillus subtilis, Saccharomyces cerevisiae and Lactobacillus acidophilus were respectively activated on the solid medium, and the above-mentioned activated microbial strains were respectively inoculated in the liquid medium, and the liquid medium was placed on a shaker at 30° C. Cultivate under the condition of 120r / min for 24 hours to obtain the culture medium of the cultured microorganisms; weigh 06L of the culture medium of Bacillus subtilis, 0.3L of the culture medium of Saccharomyces cerevisiae and 0.1L of the culture medium of Lactobacillus acidophilus, put them into the fermentation container, add molasses 2kg, corn gluten powder 200g, sodium bicarbonate 50g, urea 30g, sodium chloride 50g, add water 150L, stir evenly, ferment to obtain microbial strain activation liquid.
[0090] Weigh the following raw materials: licorice residue 160kg, traditional Chinese medicine 40kg, grain processing auxiliary materials (flax cake, soybean meal, DDGS, corn, r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com