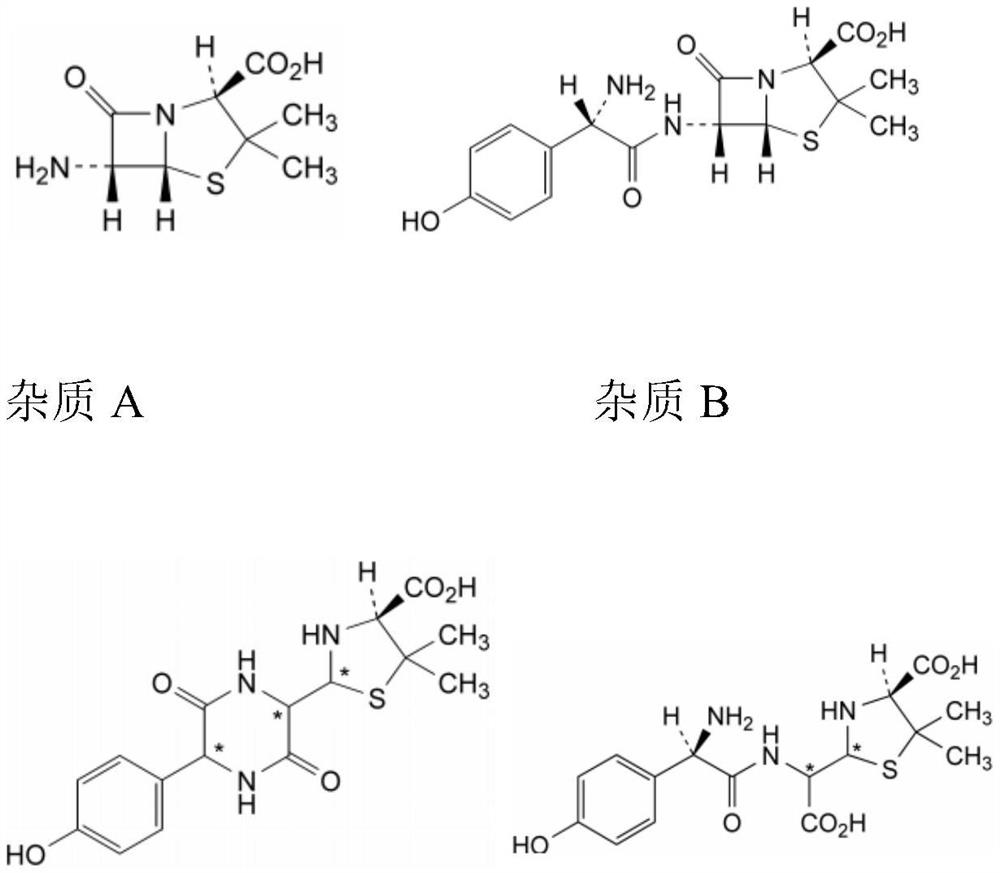
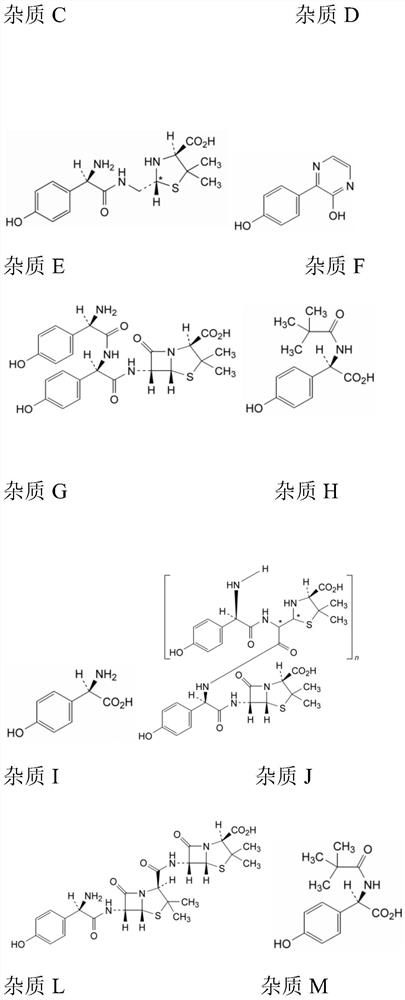
Method for detecting impurities in amoxicillin granules
A technology of amoxicillin and detection method, which is applied in the field of drug analysis, can solve the problems of few impurities, impurities that cannot be effectively separated, and cannot be separated at the same time, so as to ensure the quality of drugs, improve the separation effect of the system, and achieve good results in sensitivity and repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment one: amoxicillin particle analysis method one
[0034] Mobile phase A: ion-pair buffer (containing 0.05mol / L potassium dihydrogen phosphate, 0.01% tetrabutylammonium hydroxide (V / V), adjusting pH to 5.0 with phosphoric acid): acetonitrile 99:1
[0035] Mobile phase B: Ion-pair buffer (containing 0.05mol / L potassium dihydrogen phosphate, 0.01% tetrabutylammonium hydroxide (V / V), adjusting pH to 5.0 with phosphoric acid): acetonitrile 80:20
[0036] Column temperature: 25°C
[0037] Gradient elution: firstly use mobile phase A-mobile phase B (92:8) for isocratic elution, after the amoxicillin peak is eluted, follow the gradient elution procedure as follows: 0-35min, mobile phase A: mobile phase B volume ratio gradually becomes 0:100, gradient elution; 35-45min, mobile phase A: mobile phase B volume ratio is 0:100, isocratic elution; 45-46min, mobile phase A: mobile phase B volume ratio gradually changes 92:8, gradient elution; 46min later, mobile phase A: mob...
Embodiment 2
[0038] Embodiment two: amoxicillin particle analysis method two
[0039] Mobile phase A: ion-pair buffer (containing 0.05mol / L potassium dihydrogen phosphate, 0.1% tetrabutylammonium hydroxide (V / V), adjusting pH to 5.0 with phosphoric acid): acetonitrile 99:1
[0040] Mobile phase B: Ion pair buffer (containing 0.05mol / L potassium dihydrogen phosphate, 0.1% tetrabutylammonium hydroxide, adjusted pH to 5.0 with phosphoric acid): acetonitrile 80:20
[0041] Column temperature: 28°C
[0042] Gradient elution is the same as in Example 1.
Embodiment 3
[0043] Embodiment three: amoxicillin particle analysis method three
[0044] Mobile phase A: Ion-pair buffer (containing 0.05mol / L potassium dihydrogen phosphate, 0.5% tetrabutylammonium hydroxide (V / V), adjusting pH to 5.0 with phosphoric acid): acetonitrile 99:1
[0045] Mobile phase B: ion-pair buffer (containing 0.05mol / L potassium dihydrogen phosphate, 0.5% tetrabutylammonium hydroxide (V / V), adjusting pH to 5.0 with phosphoric acid): acetonitrile 80:20
[0046] Column temperature: 30°C
[0047] Gradient elution is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com