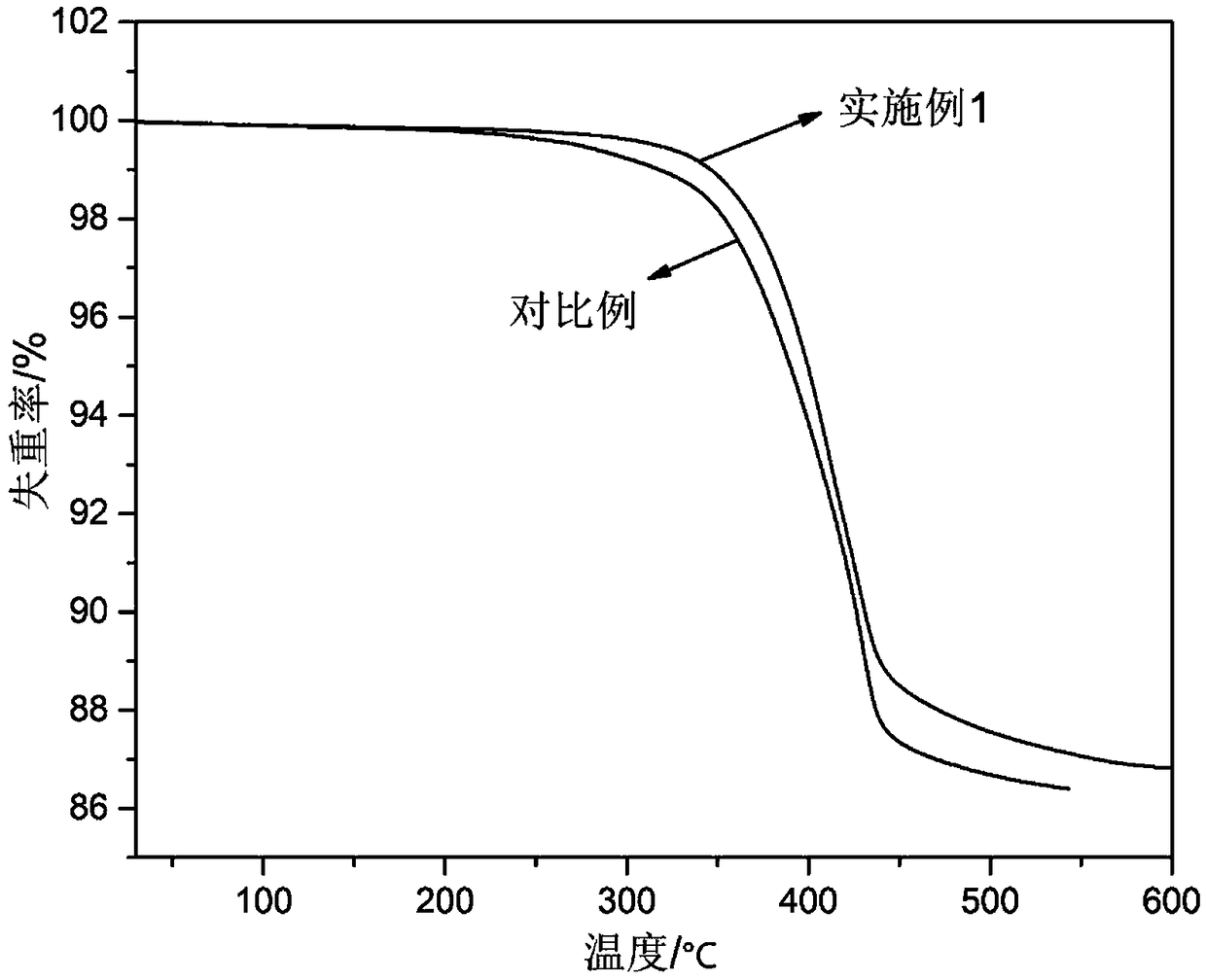
Process method for preparing zinc borate
A process method, the technology of zinc borate, applied in the directions of borates, chemical instruments and methods, boron compounds, etc., can solve the problems of difficult processing, poor thermal stability, irregular morphology, etc., to solve unstable quality, improve Excellent product quality and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 45g of zinc sulfate and 83.5g of water to prepare a solution with a content of 35% by mass, adjust the pH of the solution to 3, add it to the reactor, add 67g of borax (borax pentahydrate), 0.3g of zinc oxide , 0.03g 1-5μm zinc borate seed crystals are added to the reactor, according to the ratio of the solid reactant and water added above, add 2.9g of water to make the solid-liquid ratio of the system 1.3:1, and start the reaction at 97°C. After the reaction is kept for 5 hours, 112.33g of water is added to the reaction kettle, the temperature is naturally cooled to 50°C, and the reaction is finished after 3 hours of keeping for crystallization. The reaction liquid is suction filtered, washed, and dried at 80°C for 2 hours to obtain the product of the present invention.
Embodiment 2
[0033] Add 40g of zinc sulfate and 74.3g of water to prepare a solution with a content of 35%, adjust the pH of the solution to 2, add it to the reactor, add 70g of borax, 0.5g of zinc oxide, and 0.02g of 6-10μm Zinc borate seed crystals are added to the reaction kettle. According to the ratio of the solid reactants and water added above, 34.72g of water is added to make the solid-liquid ratio of the system 1.5:1. The temperature is raised to 95°C for the reaction, and the reaction is kept warm for 6 hours. 110.52g of water was added to the kettle, the temperature was naturally lowered to 50°C, and the reaction was completed after 2 hours of holding and crystallization. The reaction liquid is suction filtered, washed, and dried at 80°C for 2 hours to obtain the product of the present invention.
Embodiment 3
[0035] Add 50g of zinc sulfate and 93g of water to prepare a 35% solution, adjust the pH of the solution to 4, add it to the reactor, add 60g of borax, 0.1g of zinc oxide, and 0.05g of zinc borate seed crystals Add to the reactor, add 17.05g of water to make the solid-liquid ratio of the system 1:1 according to the ratio of the solid reactant and water added above, start the reaction at 102°C, keep the reaction for 4 hours, add 110.15g to the reactor The water is naturally cooled to 50°C, and the reaction is completed after 4 hours of holding and crystallization. The reaction liquid is suction filtered, washed, and dried at 80°C for 2 hours to obtain the product of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com