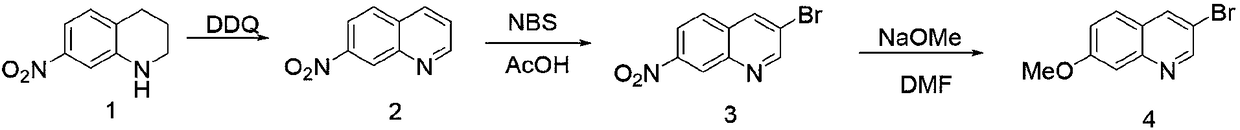
Synthetic method of 3-bromo-7-methoxyl quinoline
A technology for the synthesis of methoxyquinoline and its synthesis method, which is applied in the field of synthesis of 3-bromo-7-methoxyquinoline, and can solve the problem of instability of 2-bromomalondialdehyde, instability of preparation raw materials, troublesome post-processing, etc. problem, to achieve the effect of solving the instability of raw materials, easy enlargement, convenient operation and post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0033] The first step: the synthesis of 7-nitroquinoline
[0034] Dichloromethane (4.5L) was added to 7-nitro-1,2,3,4 tetrahydroquinoline (60g, 0.337mol), and dichlorodicyanobenzoquinone (152.9g, 0.6734mol ), stirred at room temperature for 1 h, and suction filtered, and the filter cake was stirred with dichloromethane (500 mL*2) for 5 min, and suction filtered; the combined filtrates were washed with 10% NaOH (500 mL*2), and then washed with saturated NaCl solution (500 mL), Organic phase anhydrous Na 2 SO 4 Dry and concentrate to dryness by rotary evaporation. The concentrated orange-yellow solid was stirred with 250 ml of petroleum ether: ethyl acetate = 5:1 solvent for 30 min, filtered and dried to obtain 7-nitroquinoline (53 g, 90.3%).
[0035] The second step: the synthesis of 3-bromo-7-nitroquinoline
[0036] Glacial acetic acid (350ml) was added to 7-nitroquinoline (50g, 0.287mol), the temperature was raised to 106°C, and N-bromosuccinimide (51.1g, 0.2870mol) was a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com