IR820-PTX amphiphilic small-molecule prodrug in combination of photothermal therapy and chemotherapy as well as nano-particle preparation method and application thereof
A technology of IR820-PTX and IR820-COOH is applied in the field of medicine to achieve anti-tumor effect, low toxicity and solve the effect of poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 IR820-PTX prodrug molecular synthesis
[0039] Precisely weigh a certain amount of IR820 with an analytical balance in a 100mL round-bottom flask, add anhydrous DMF to dissolve it, accurately weigh 6-aminocaproic acid in anhydrous DMF and ultrasonically mix it into the round-bottom flask, add to the mixed solution Triethylamine was reacted in an oil bath at 85°C for 4 h under the protection of nitrogen. After the reaction was complete, the anhydrous DMF in the filtrate was removed by suction filtration under reduced pressure, and the crude product was dried overnight in a vacuum oven. The dried product was redissolved in methanol, the sample was mixed with silica gel, evaporated to dryness, and the solid was separated by silica gel column, eluted with ethyl acetate and methanol gradient, and the dried IR820-COOH was collected. Precisely weigh a certain amount of IR820-COOH in a 100mL round-bottomed flask with an analytical balance, add anhydrous DMF to diss...
Embodiment 2
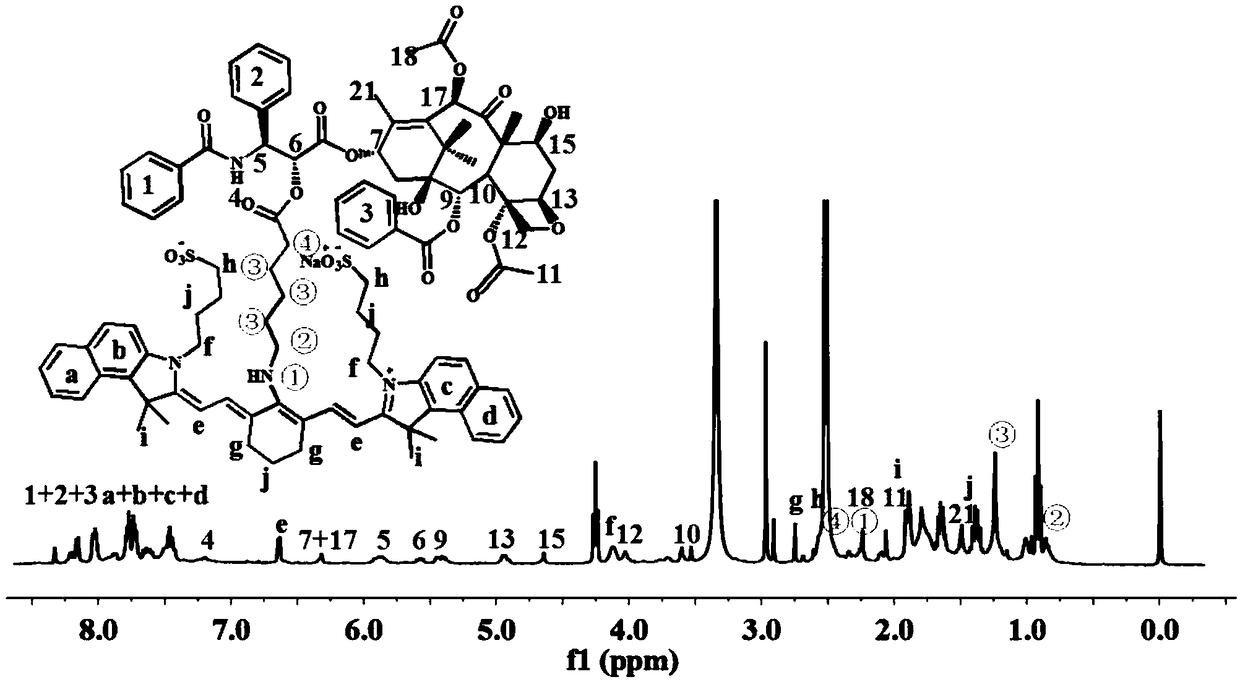
[0040] Embodiment 2 Proton nuclear magnetic resonance spectrum ( 1 H-NMR) identification of IR820-PTX prodrug chemical structure
[0041] Weigh about 5 mg of the IR820-PTX prodrug, dissolve it in deuterated dimethyl sulfoxide (DMSO-d6) and place it in an NMR tube, use 400MHz H NMR spectrum to measure its H NMR spectrum, using tetramethylsilane as internal standard Record the chemical shift value (ppm) of the compound. The result is as figure 1 As shown, the NMR results can confirm that the peaks of IR820 and PTX in the newly synthesized molecule can be assigned, and the chemical shift of the typical PTX reaction hydroxyl group appears, which proves the synthesis of the ester bond. pass 1 H-NMR spectrum can confirm the successful synthesis of IR820-PTX prodrug.
Embodiment 3
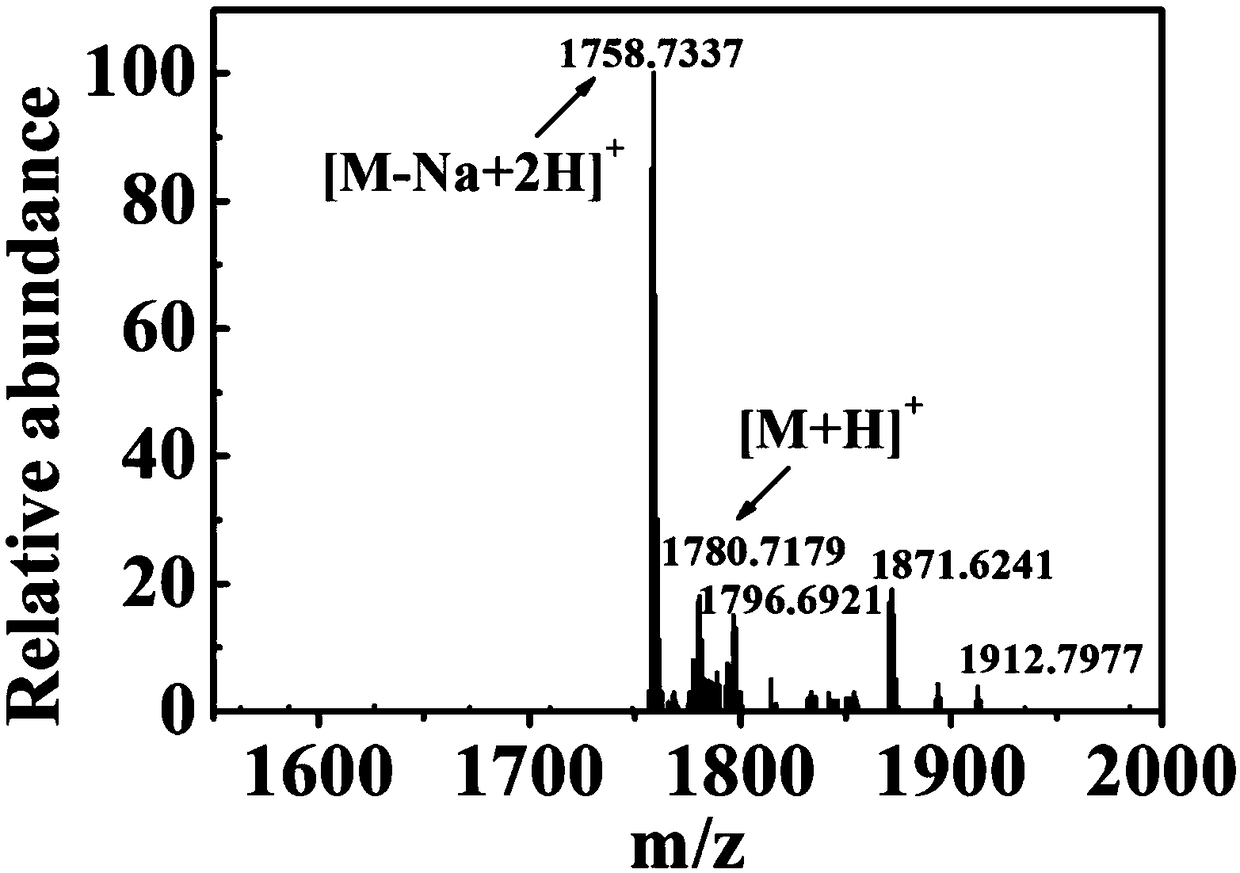
[0042] Example 3 High-resolution mass spectrometry (HRMS) identification of IR820-PTX prodrug chemical structure
[0043] About 1 mg of IR820-PTX prodrug was weighed, dissolved in methanol, and analyzed by high-resolution mass spectrometry. The result is as figure 2 As shown, the mass spectrometry results can be seen, the molecular ion peak [M+H] + (1780.7179) and [M-Na+2H] + The appearance of (1758.7377) proves the successful synthesis of IR820-PTX prodrug
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com