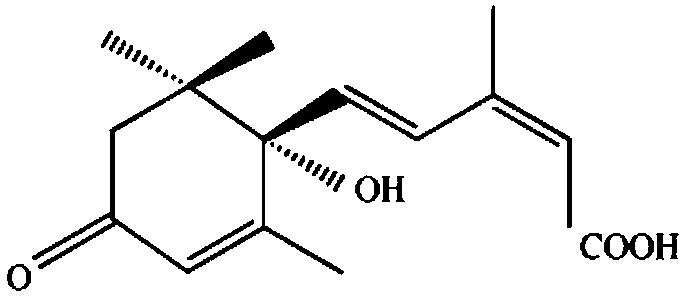
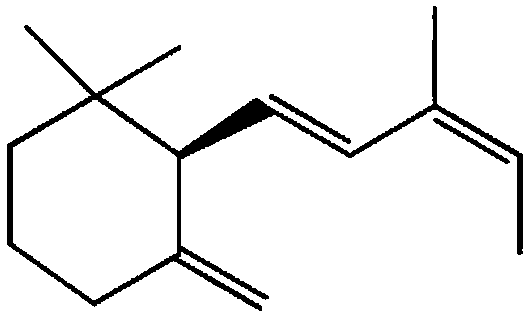
Sesquiterpene cyclase, preparation and application thereof, and synthesis method of 2Z,4E-alpha-ionylideneehane
A technology of terpene cyclase and application method, applied in the fields of microbial chemistry and natural product chemistry, can solve the problems of high cost, low yield, low efficiency of natural ABA and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
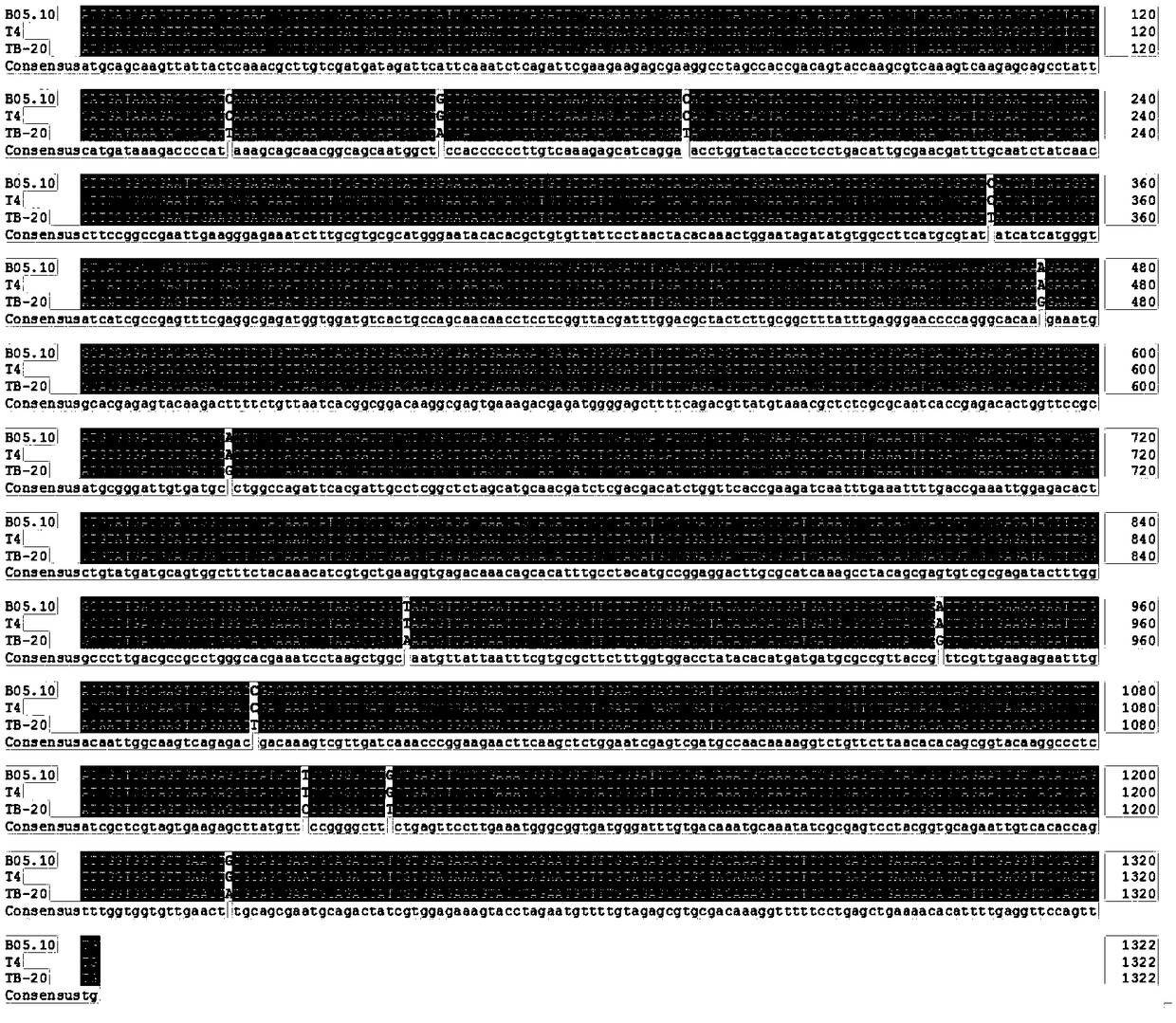
Image
Examples
Embodiment 1
[0047] Cloning of the gene encoding the sesquiterpene cyclase BcABA3.
[0048] 1. Culture of B.cinerea TBC-20 bacteria
[0049] Take a single colony slant of TBC-20 (the height of the slant is about 8cm, at 26°C, and the PDA medium is cultured for 7 days and then stored at 4°C), suck about 5ml of sterilized water with a dropper, and scratch the spores on the slant. Mix the mixture of spores several times, pipette 2ml into a 250ml Erlenmeyer flask containing 50ml of culture medium with a dropper, and place it on a shaker at 26°C and cultivate at 180rpm. After culturing for about 50h, the seed liquid was inoculated into a 250ml Erlenmeyer flask containing 50ml of culture medium with a 5ml pipette at a ratio of 5% of the inoculum, and cultured at 26°C and 180rpm. The medium consists of 6.0g / L glucose, 10g / L yeast extract, 3.0g / L soluble starch, 1.0g / L soybean flour, 2.0g / L sucrose, 0.5g / L NH 4 NO 3 and 1.0g / L KH 2 PO 4 composition.
[0050] 2. Extraction of genomic DNA from...
Embodiment 2
[0064] Construction of expression vector for sesquiterpene cyclase BcABA3.
[0065] The endonuclease cleavage sites NcoI and XhoI contained in the base sequence of the sesquiterpene cyclase BcABA3 obtained in Example 1 were determined.
[0066] The pET28a(+) plasmid (stored in the laboratory) was selected as the vector required for expressing the sesquiterpene cyclase BcABA3. Compare the enzyme cleavage site contained in the corresponding base sequence of the sesquiterpene cyclase BcABA3 with the enzyme cleavage site in the multi-cloning site region of the vector, and select the multi-cloning site region of the expression vector that contains the cleavage site. The NcoI and XhoI sites that are not in the base sequence of hemiterpene cyclase BcABA3 are used as restriction sites for constructing plasmids. See Table 2 for primers designed to contain restriction sites. PCR amplification was performed using KODplus-neo enzyme (purchased from Toyobo Co., Ltd.).
[0067] Table 2 S...
Embodiment 3
[0072] Linearization of the E. coli expression plasmid and transformation of E. coli to obtain a transformant containing the gene whose base sequence is shown in SEQ ID No. 1.
[0073] The recombinant plasmid pET-28(a)-SC3 obtained in Example 2 was transformed into competent cells of Escherichia coli E.coli BL21(DE3) strain (preserved in the laboratory) (the competent cells were prepared by the Inoue method). The specific operations are as follows:
[0074] 1) Pick a small amount of Transgene frozen E.coli DH5α cells stored at -80°C with a Tip, then streak the LB plate without antibiotics, and place the plate upside down in a bacterial incubator for 16 hours.
[0075] 2) Pick a newly activated E.coli DH5α single colony (2-3mm in diameter) from the LB plate and inoculate it in 3mL SOB liquid medium (add MgCl before use) 2 ), 37°C, 250rpm shaking culture for 7 hours, record the OD value.
[0076] 3) The bacterial suspension grown to the late logarithmic growth phase was inocul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com