Preparation method of a photoelectrochemical sensor for detecting glutathione
A photoelectrochemical and glutathione technology, applied in the direction of material electrochemical variables, etc., to achieve high sensitivity and photoelectrochemical signal enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
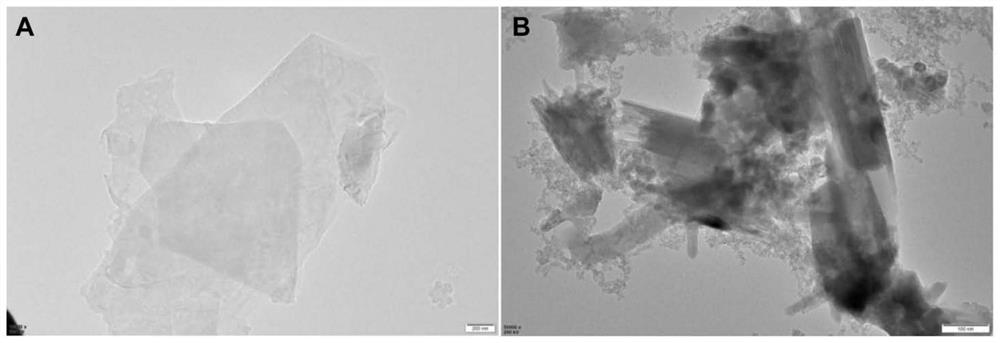
[0026] (1) Preparation and stripping of tin selenide
[0027] a. Preparation: Take 0.6769g tin dichloride (SnCl 2 ﹒ 2H 2 O) and 0.2368g solid tin powder (Se) in a beaker, measure 48mL ethylene glycol with a measuring cylinder and pour it into the beaker, then measure 12mL ethylenediamine and pour it into the beaker, and stir for 30 minutes, then pour it into In the reaction kettle, put it in an oven at 160°C, and take it out after 12 hours to obtain tin selenide (SnSe);
[0028] b. Stripping: Mix the prepared SnSe solution with N,N-dimethylformamide (DMF) according to the volume ratio of 1:1 and place it in a centrifuge tube for ultrasonication for 2 hours; take out the ultrasonicated solution and centrifuge , and washed 3 times with absolute ethanol, then added deionized water to disperse to form a 2mg / mL solution for later use.
[0029] (2) Exfoliation of hydrotalcite-like
[0030] a. Preparation. According to the molar ratio of Mg-Al is 3:1 will AlCl 3 ·6H 2 O and M...
Embodiment 2
[0037] Example 2 Potential optimization
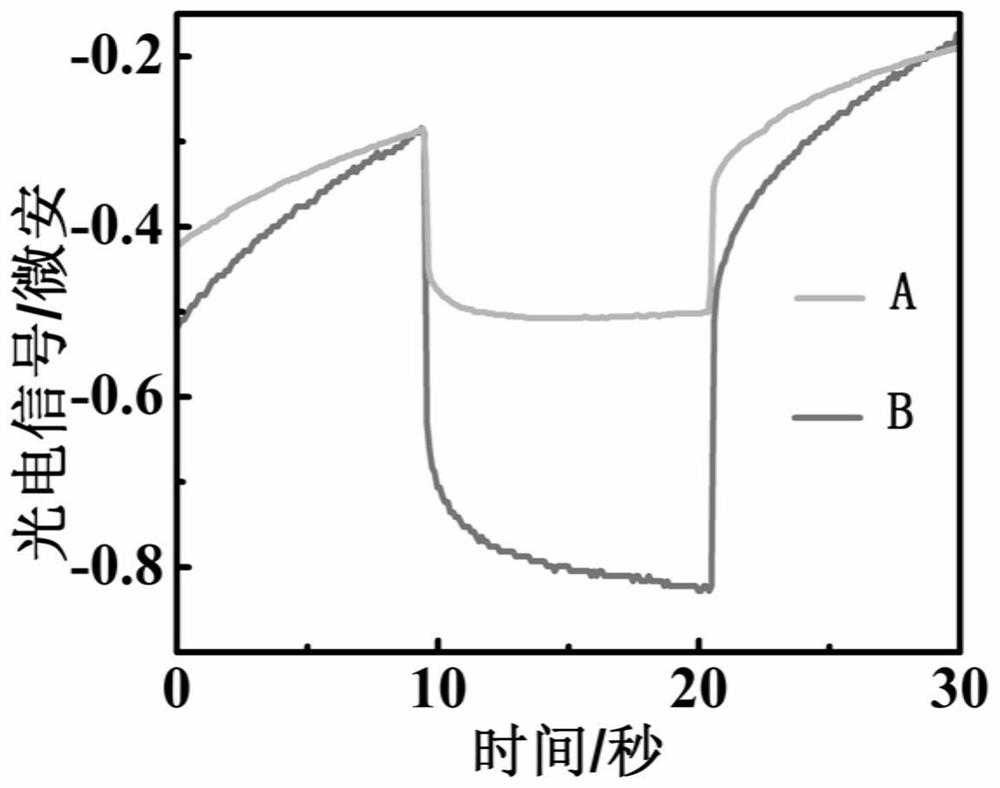
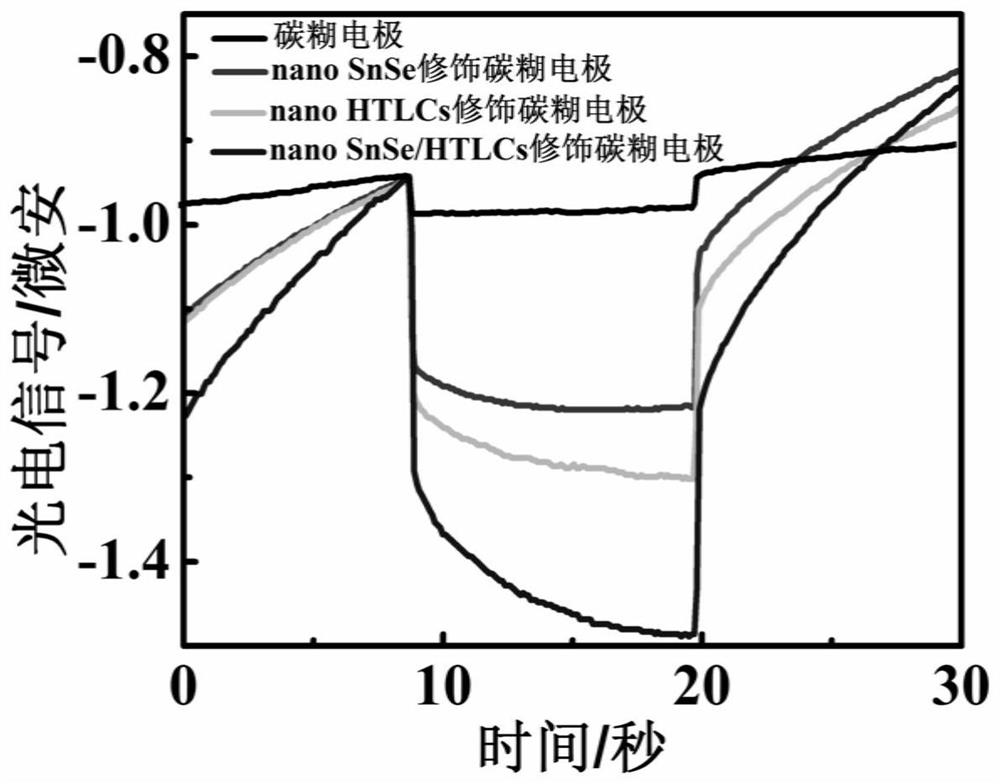
[0038] The prepared photochemical sensor will change its own electron transfer system due to the difference in electrode potential, which will have a certain impact on the measurement of photoelectrochemical signals. The photoelectrochemical signals were measured at different working potentials of -0.1V, 0.0V, 0.1V and 0.2V, respectively. The experimental results show that with the increase of the electrode potential, the photoelectrochemical signal value tends to increase. But as the potential increases, the peak shape is poor when the potential is 0.2V. When measuring at an electrode potential of 0.1V, the photoelectrochemical signal value is relatively large and the peak shape is relatively good for reading, so the final electrode potential is 0.1V.
Embodiment 3
[0039] Example 3 pH optimization
[0040] The electrodes were placed in 10 -6 In the glutathione solution of M, the photoelectrochemical signal was measured under the conditions of pH 6.0, 6.5, 7.0, 7.5, and 8.0. The experimental results show that under the condition of potential 0.1V, the photoelectrochemical signal response measured under different pH conditions. It can be seen that the signal increases when the pH is 6.0-7.0, reaches the maximum value when the pH is around 7.0, and shows a downward trend in the range of 7.0-8.0. Therefore, the pH of the detection medium is chosen to be 7.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com