8-hydroxyquinoline type compounds and preparation method thereof
A hydroxyquinoline and compound technology, which is applied in the field of 8-hydroxyquinoline compounds and their preparation, can solve the problems of high corrosiveness of chlorine-containing organic substances, high toxicity of o-chloroaniline, environmental pollution by tar, etc., and is conducive to rapid construction , The effect of low catalyst dosage and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
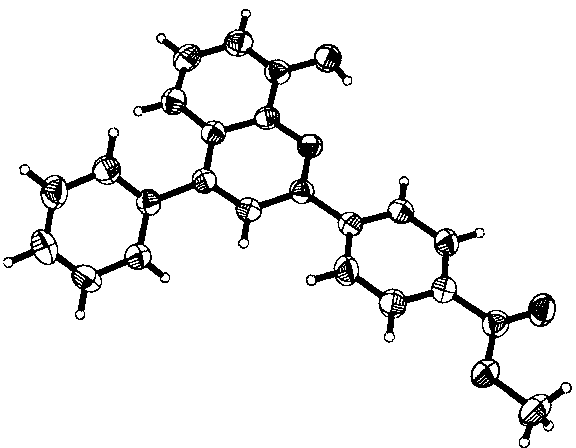
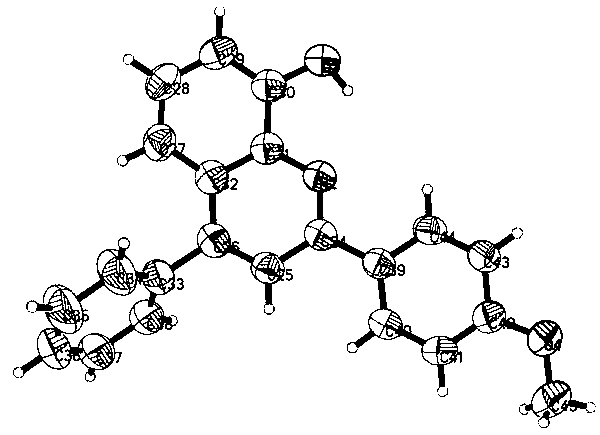
Image
Examples
Embodiment 8
[0029] The synthetic method of present embodiment 8-hydroxyquinoline compound 4a is as follows:
[0030]
[0031] Add o-aminophenol (1.1mmol), benzaldehyde (1mmol) and phenylacetylene (1.2mmol) successively to a solution of AgOTf (0.5mol%) in dichloroethane (4mL), stir at room temperature, then add trifluoroacetic acid (4mmol), the temperature was raised to 80°C for reaction. TLC monitored the reaction until it was complete. After adding 20 mL of dichloromethane, washed with water, dried over anhydrous sodium sulfate, and separated by column chromatography (PE / EA=20:1 as the eluent), 280 mg of light yellow solid powder was obtained, with a yield of 94%.
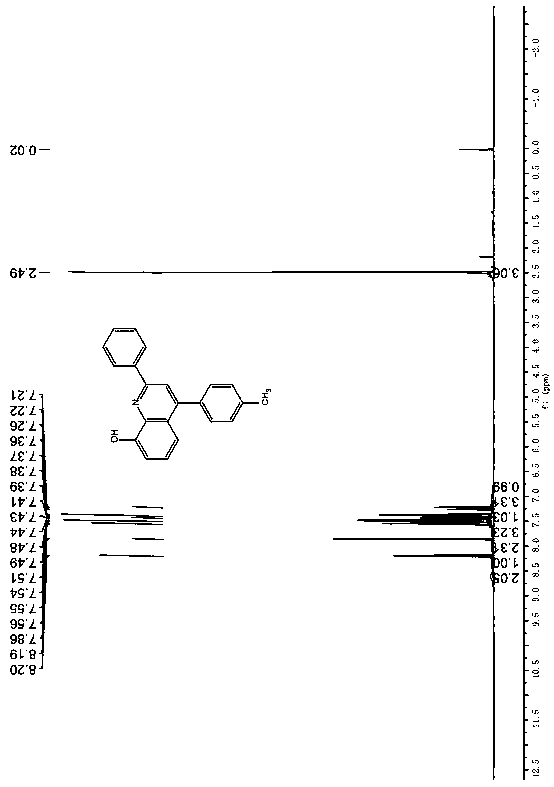
[0032] 1 H NMR (600MHz, CDCl 3 )δ8.16-8.20 (m, 2H), 7.85 (s, 1H), 7.46-7.58 (m, 8H), 7.37-7.40 (m, 2H), 7.21 (d, J = 8.7Hz, 1H) ppm; 13 C NMR (150MHz, CDCl 3 )δ152.0, 150.2, 147.3, 136.4, 136.2, 135.8, 127.2, 127.1, 126.9, 126.5, 126.1, 125.8, 125.0, 124.8, 123.2, 123.6, 123.5, 117.5, 113.6, 107.6ppm.
Embodiment 2
[0034] The synthetic method of present embodiment 8-hydroxyquinoline compound 4b is as follows:
[0035]
[0036] Add o-aminophenol (1.2mmol), p-tolualdehyde (1mmol) and phenylacetylene (1.3mmol) successively to the toluene (4mL) solution containing AgOTf (0.5mol%), after stirring at room temperature, add trifluoroacetic acid (3mmol), the temperature was raised to 80°C for reaction. Followed by TLC spotting until the reaction was complete, concentrated to remove toluene, added 20 mL of dichloromethane, washed with water, dried over anhydrous sodium sulfate, and separated by column chromatography (PE / EA=20:1 as eluent) to obtain 286 mg of light yellow solid , yield 92%.
[0037] 1 H NMR (600MHz, CDCl 3 )δ8.09(d,J=8.2Hz,2H),7.84(s,1H),7.55(ddd,J=22.1,11.4,4.4Hz,5H),7.33-7.39(m,4H),7.20(dd ,J=6.1,2.6Hz,1H),2.45(s,3H)ppm; 13 C NMR (150MHz, CDCl 3 )δ153.4, 151.4, 148.5, 138.8, 137.5, 137.3, 134.9, 128.7, 128.6, 128.4, 128.3, 127.6, 127.5, 126.4, 126.2, 126.1, 124.8, 118.7,...
Embodiment 3
[0039] The synthetic method of present embodiment 8-hydroxyquinoline compound 4c is as follows:
[0040]
[0041]Add o-aminophenol (1.1mmol), 2,4,6-trimethylbenzaldehyde (1mmol) and phenylacetylene (1.5mmol) successively to a solution of AgOTf (1mol%) in dioxane (4mL), room temperature After stirring evenly, trifluoroacetic acid (1 mmol) was added, and the temperature was raised to 110°C for reaction. Tracked by TLC until the reaction was complete, concentrated to remove dioxane, added 20mL of dichloromethane, washed with water, dried over anhydrous sodium sulfate, and separated by column chromatography (PE / EA=15:1 as eluent) to obtain light Yellow solid 298mg, yield 88%.
[0042] 1 H NMR (600MHz, CDCl 3 )δ7.55-7.58(m,2H),7.53(t,J=7.3Hz,2H),7.47-7.51(m,2H),7.41-7.45(m,1H),7.35(s,1H),7.19 -7.22(m,1H),7.01(s,2H),2.37(s,3H),2.11(s,6H)ppm; 13 C NMR (150MHz, CDCl3) δ156.6, 151.5, 147.9, 137.0, 136.9, 134.8, 128.5, 127.5, 127.5, 127.4 126.4, 124.4, 108.7, 20.1, 19.3ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com