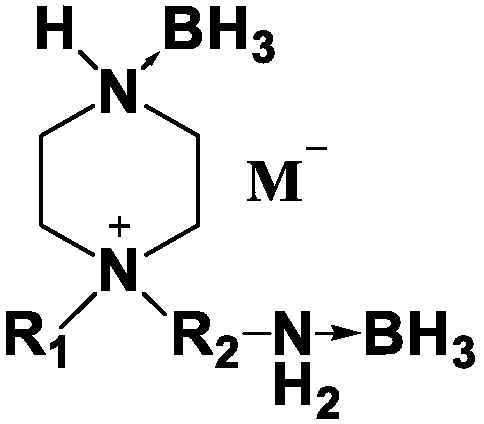
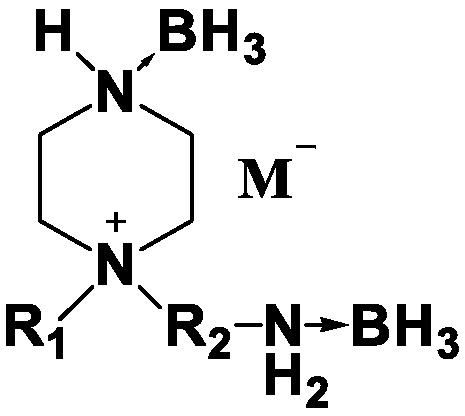
Piperazine cation-base ionic liquid containing ammonia and boron groups and preparation method thereof
A cation-based and ionic liquid technology, which is applied in the field of piperazine cation-based ionic liquids and their preparation, can solve the problems of low specific impulse of ionic liquids, and achieve the effects of low cost, easy availability of raw materials, violent and long-lasting combustion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Dissolve 1.78g of sodium dicyanamide (89g / mol) in water, add 4.38g of silver nitrate (219g / mol), and stir magnetically until a large amount of white solid silver dicyanamide appears in the solution. After the solution is suction filtered, the white solid dicyandiamide Amine silver protected from light for later use.
[0022] Add 30ml of methanol, 0.95g of N-methylpiperazine (100.16g / mol), 2.1g of 2-bromoethylamine hydrobromide (204.89g / mol) into a 100ml single-necked bottle, stir magnetically, and react at room temperature for 15 hours. The solution was extracted several times with dichloromethane to give the piperazinium salt as a liquid. Add 25ml of borane tetrahydrofuran solution with a borane concentration of 1.0M, stir magnetically, and heat in an oil bath at 75°C for 10h. After returning to room temperature, the tetrahydrofuran solution was spin-dried to obtain a borane complex of piperazinium salt. Dissolve the obtained piperazinium salt borane complex in 30ml ...
Embodiment 2
[0024] Dissolve 1.78g of sodium dicyanamide (89g / mol) in water, add 4.38g of silver nitrate (219g / mol), and stir magnetically until a large amount of white solid silver dicyanamide appears in the solution. After the solution is suction filtered, the white solid dicyandiamide Amine silver protected from light for later use.
[0025] Add 35ml of methanol, 1.71g of N-ethylpiperazine (114.16g / mol), 2.41g of 3-bromopropylamine hydrobromide (218.92g / mol) in a 100ml single-necked bottle, magnetically stir, and react at room temperature for 18 hours. The solution was extracted several times with dichloromethane to give the piperazinium salt as a liquid. Add 25ml of borane tetrahydrofuran solution with a borane concentration of 1.0M, stir magnetically, and heat in an oil bath at 75°C for 12h. After returning to room temperature, the tetrahydrofuran solution was spin-dried to obtain a borane complex of piperazinium salt. Dissolve the obtained piperazinium salt borane complex in 30ml o...
Embodiment 3
[0027]Under magnetic stirring at 0-5°C, add 3.73 g of potassium hydroxide (dissolved in 25 ml of ethanol) dropwise into 60 ml of ethanol solution of 8.64 g of dinitroaminopropionitrile, stir at 20°C for 30-40min, then cool to 0°C , Filtrated to obtain 5.3g potassium dinitramide (131g / mol). Dissolve potassium dinitramide in water, add 8.96 g of silver nitrate (219 g / mol), stir magnetically until a large amount of white solid silver dinitrate appears in the solution, and filter the solution to avoid light for later use.
[0028] Add 35ml of methanol, 1.71g of N-methylpiperazine (100.16g / mol), 2.41g of 3-bromopropylamine hydrobromide (218.92g / mol) successively in a 100ml single-necked bottle, stir magnetically, and react at room temperature for 15h. The solution was extracted several times with dichloromethane to give the piperazinium salt as a liquid. Add 25ml of borane tetrahydrofuran solution with a borane concentration of 1.0M, stir magnetically, and heat in an oil bath at 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com