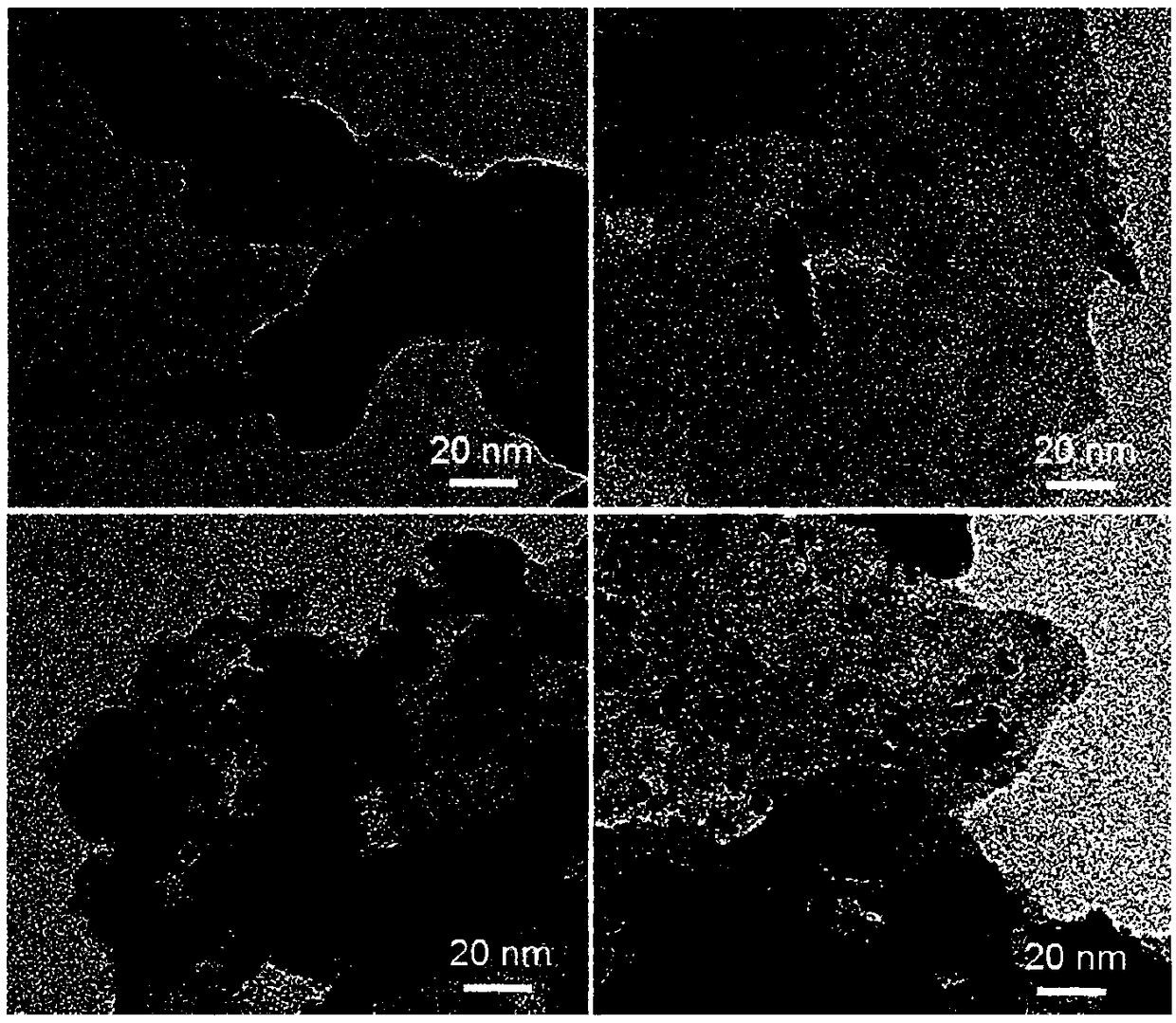
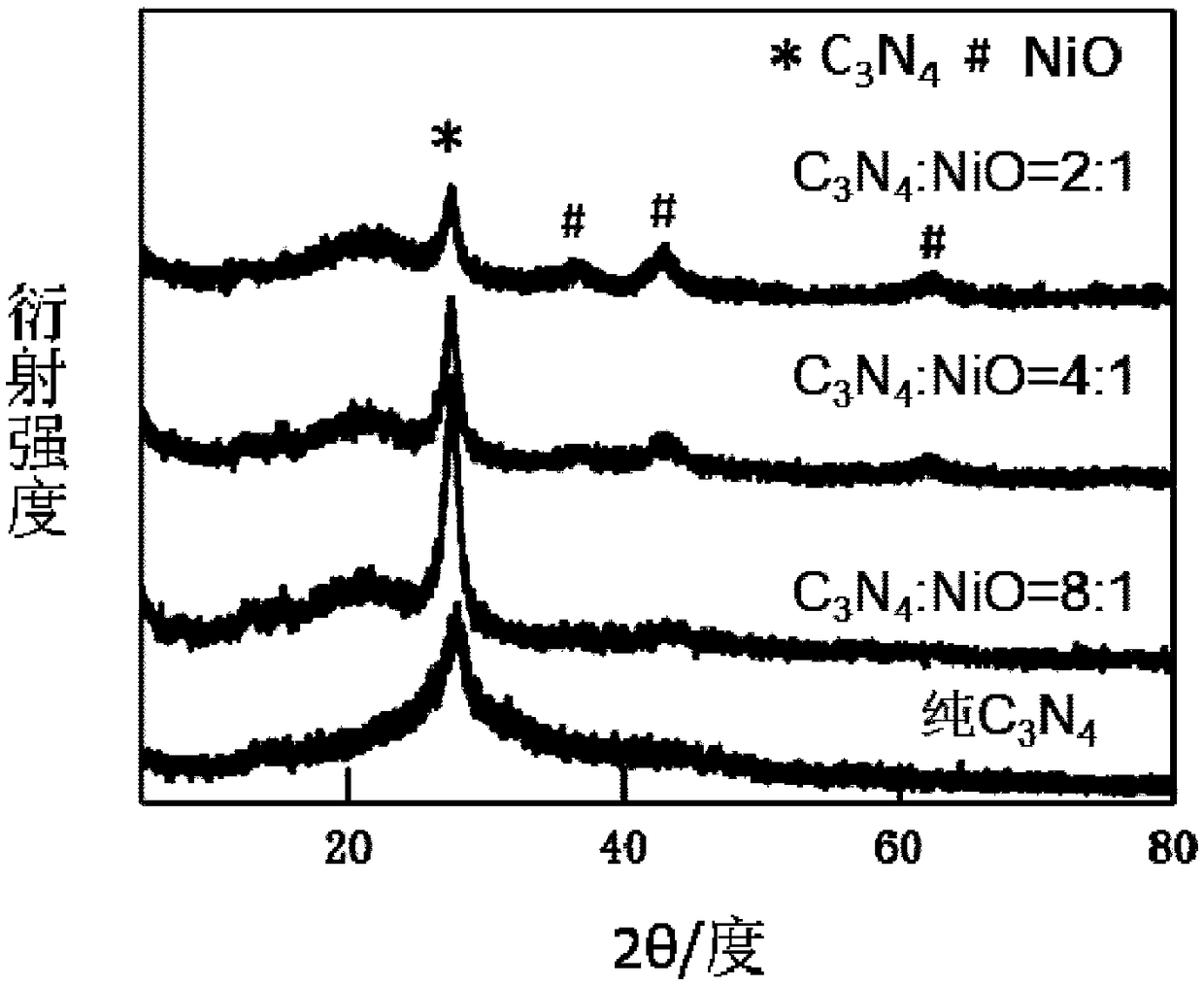
Preparation method of nickel oxide-carbon nitride composite photocatalyst
A carbon nitride, nickel oxide technology, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of low photocatalytic performance, carrier recombination, and low quantum efficiency, etc. To achieve the effect of simple process, good repeatability, and improved photocatalytic hydrogen production rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1) Fill a corundum crucible with 36g of urea and heat it in an oven at 80°C for 24 hours, then cover it and heat it up to 550°C in a muffle furnace at a rate of 4.6°C / min, and keep it warm for 3 hours. Cooling to room temperature, a yellow powdery solid C 3 N 4 , ready to use after grinding;
[0022] 2) Add 184mg of C to the screw bottle 3 N 4 powder, then add 30mL of tert-butanol, and ultrasonically disperse at 40°C for 0.5h to obtain dispersion A; Stir on the stirrer for 12h to obtain the mixed solution B;
[0023] 3) Preheat a 50mL reactor with a polytetrafluoroethylene liner to 70°C, then quickly transfer the mixed solution B to the polytetrafluoroethylene liner, quickly close the reactor and transfer it to temperature In an oven at 100°C, then set the oven temperature to 210°C, when the temperature rises to the set temperature, start timing, and react at constant temperature for 24 hours;
[0024] 4) After the solvothermal reaction is over, turn off the power...
Embodiment 2
[0026] 1) Fill a corundum crucible with 36g of urea and heat it in an oven at 80°C for 24 hours, then cover it and heat it up to 550°C in a muffle furnace at a rate of 4.6°C / min, and keep it warm for 3 hours. Cooling to room temperature, a yellow powdery solid C 3 N 4 , ready to use after grinding;
[0027] 2) Add 184mg of C to the screw bottle 3 N 4 powder, then add 30mL of tert-butanol, ultrasonically disperse at 40°C for 0.5h to obtain dispersion A; then add 128mg of nickel acetylacetonate to dispersion A, then ultrasonically disperse at 40°C for 0.5h Stir on the stirrer for 12h to obtain the mixed solution B;
[0028] 3) Preheat a 50mL reactor with a polytetrafluoroethylene liner to 70°C, then quickly transfer the mixed solution B to the polytetrafluoroethylene liner, quickly close the reactor and transfer it to temperature In an oven at 100°C, then set the oven temperature to 210°C, when the temperature rises to the set temperature, start timing, and react at constan...
Embodiment 3
[0031] 1) Fill a corundum crucible with 36g of urea and heat it in an oven at 80°C for 24 hours, then cover it and heat it up to 550°C in a muffle furnace at a rate of 4.6°C / min, and keep it warm for 3 hours. Cooling to room temperature, a yellow powdery solid C 3 N 4, ready to use after grinding;
[0032] 2) Add 184mg of C to the screw bottle 3 N 4 powder, then add 30mL of tert-butanol, ultrasonically disperse at 40°C for 0.5h to obtain dispersion A; then add 257mg of nickel acetylacetonate to dispersion A, then ultrasonically disperse at 40°C for 0.5h Stir on the stirrer for 12h to obtain the mixed solution B;
[0033] 3) Preheat a 50mL reactor with a polytetrafluoroethylene liner to 70°C, then quickly transfer the mixed solution B to the polytetrafluoroethylene liner, quickly close the reactor and transfer it to temperature In an oven at 100°C, then set the oven temperature to 210°C, when the temperature rises to the set temperature, start timing, and react at constant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com