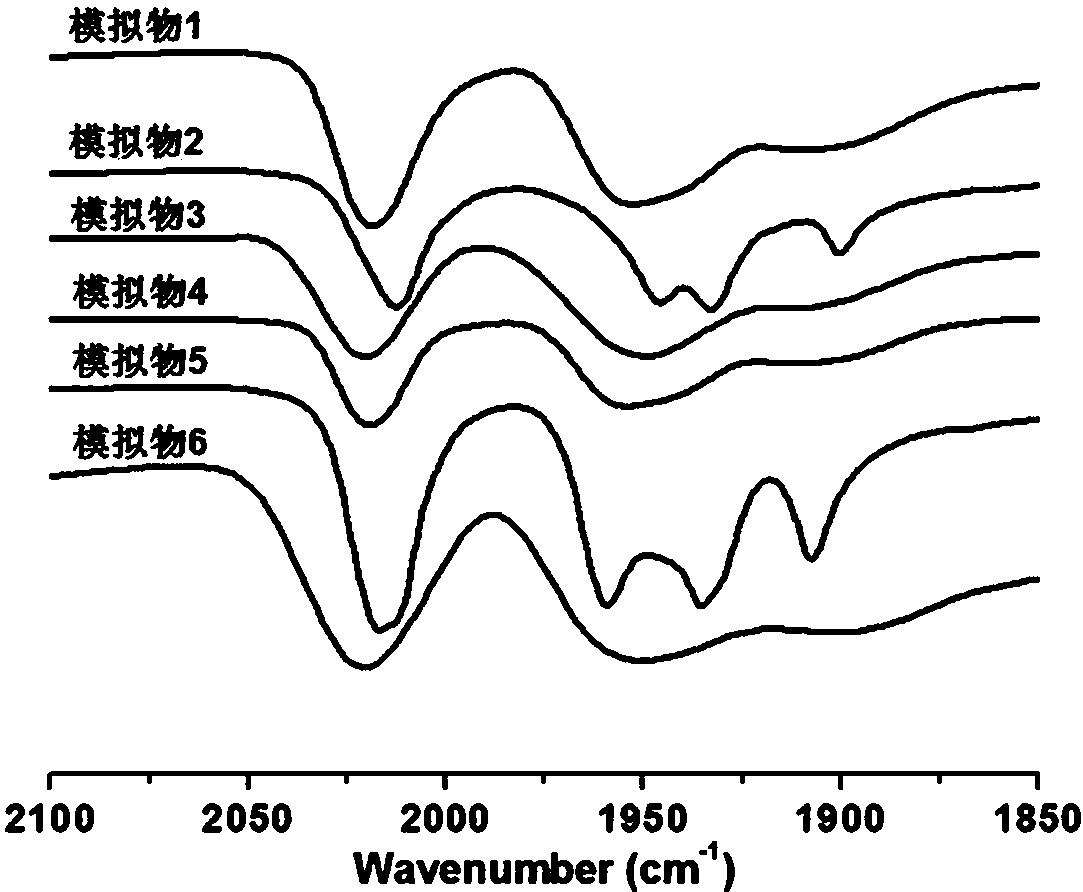
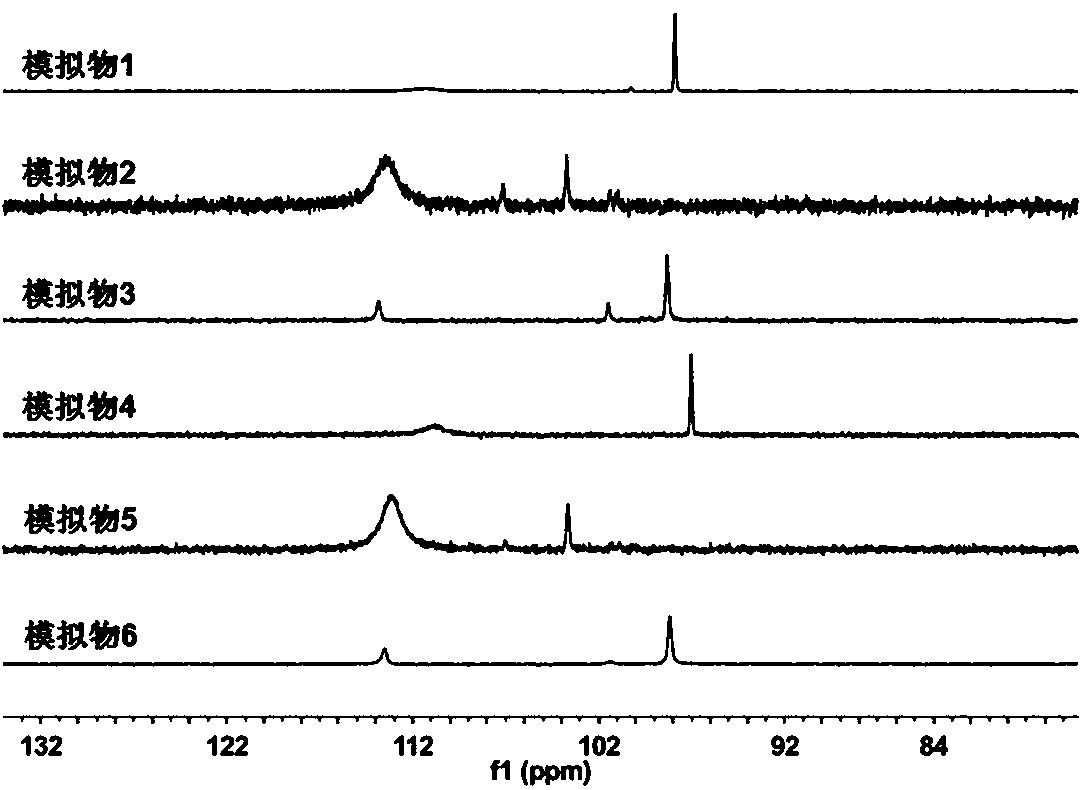
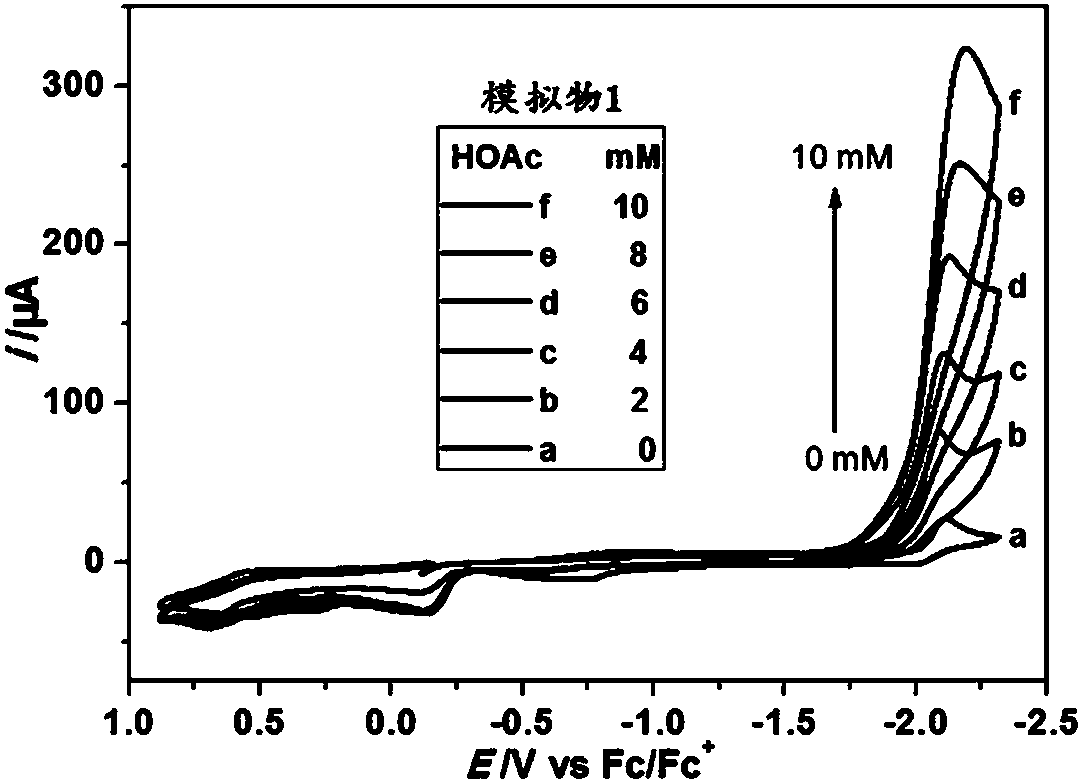
Asymmetrically substituted Fe-Fe hydrogenase mimics, photochemical synthesis method and application thereof
A technology for photochemical synthesis and iron hydrogenase, which is applied in the fields of iron organic compounds, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., to achieve the effects of simple preparation operation, high reaction rate and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Photochemical synthesis of aminobisphosphine chelated substituted iron-iron hydrogenase mimic 1 containing azapropylene group, whose chemical formula is Fe 2 (μ-SCH 2 N(Ph)CH 2 S-μ)(CO) 4 {k 2 -(Ph 2 P) 2 N(CH 2 ) 3 NMe 2}, and its preparation process is as follows:
[0038]
[0039] Its concrete preparation steps are as follows:
[0040] 0.070g (0.15mmol) Fe 2 {μ-SCH 2 N(Ph)CH 2 S-μ}(CO) 6 and 0.085g (0.18mmol, 1.2 equivalents) (Ph 2 P) 2 N(CH 2 CH 2 CH 2 NMe 2 ) mixture was added to a long Schlenk bottle with a stirring magnet, and after the nitrogen was replaced 3 times, 90mL of toluene was injected and stirred to dissolve to obtain a light red solution; under the condition of avoiding light, the power was 20W and the wavelength was 365nm The above-mentioned mixed solution was irradiated vertically with a special LED lamp, and irradiated at room temperature for 2 hours. The red solution turned into a black-red solution. TLC monitored the disappe...
Embodiment 2
[0047] Photochemical synthesis of ethylene-containing aminobisphosphine chelated substituted iron-iron hydrogenase mimetic 2 with the chemical formula Fe 2 (μ-SCH 2 CH 2 S-μ)(CO) 4 {k 2 -(Ph 2 P) 2 N(CH 2 ) 3 NMe 2}, and its preparation process is as follows:
[0048]
[0049] Its concrete preparation steps are as follows:
[0050] 0.930g (0.25mmol) Fe 2 {μ-SCH 2 CH 2 S-μ}(CO) 6 and 0.142g (0.30mmol, 1.2 equivalents) (Ph 2 P) 2 N(CH 2 CH 2 CH 2 NMe 2 ) was added to a long-shaped Schlenk bottle with a stirring magnet, and after the nitrogen gas was replaced 3 times, 100 mL of toluene was injected and stirred to dissolve to obtain a light red solution; under the condition of avoiding light, the power was 20W and the wavelength was 365nm The above-mentioned mixed solution was irradiated vertically by a special LED lamp, and irradiated at room temperature for 3 hours. The red solution turned into a black-red solution. TLC monitored the disappearance of the re...
Embodiment 3
[0057] Photochemical synthesis of oxapropylene group-containing aminobisphosphine chelated substituted iron-iron hydrogenase mimic 3, whose chemical formula is Fe 2 (μ-SCH 2 OCH 2 S-μ)(CO) 4 {k 2 -(Ph 2 P) 2 N(CH 2 ) 3 NMe 2}, and its preparation process is as follows:
[0058]
[0059] Its concrete preparation steps are as follows:
[0060] 0.059g (0.15mmol) Fe 2 {μ-SCH 2 OCH 2 S-μ}(CO) 6 and 0.085g (0.18mmol, 1.2 equivalents) (Ph 2 P) 2 N(CH 2 CH 2 CH 2 NMe 2 ) mixture was added to a long Schlenk bottle with a stirring magnet, and after the nitrogen was replaced 3 times, 90mL of toluene was injected and stirred to dissolve to obtain a light red solution; under the condition of avoiding light, the power was 20W and the wavelength was 365nm The above-mentioned mixed solution was irradiated vertically with a special LED lamp, and irradiated at room temperature for 2 hours. The red solution turned into a black-red solution. TLC monitored that the reaction r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com