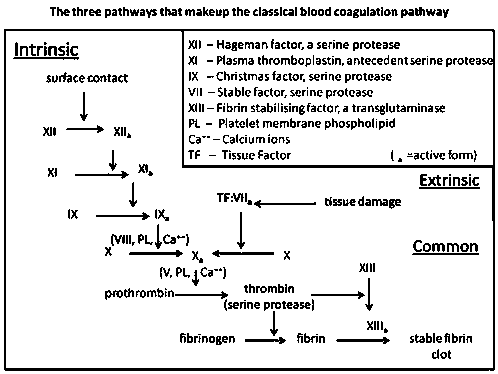
Procoagulant polypeptide LGTX-F2 and application thereof
A LGTX-F2, coagulation-promoting technology, applied in the field of biomedicine, can solve the problems of insufficient understanding of important functions, lack of hemostatic drugs, and no in-depth research on active ingredients, etc., and achieve obvious effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Preparation of procoagulant polypeptide LGTX-F2
[0018] Construction of prokaryotic expression vector
[0019] Select the appropriate prokaryotic expression vector according to the size of the target protein to facilitate the subsequent separation and purification steps. Finally, it was determined to use the PET-32a prokaryotic expression vector to express the protein. Considering the high expression efficiency of the vector and the fusion protein (adapter protein 18KDa + target protein 7KDa) digested by TEV, the molecular weight difference between the adapter protein and the target protein is relatively large, which is easy for subsequent separation and purification. Finally, the double restriction site of the prokaryotic expression vector was determined to use EcoR I (-GG^AATTCC-) and Hind III (-CA^AGCTTG-) two restriction sites, and the rTEV restriction site (-ENLYFQ ^G-), the prokaryotic expression vector plasmid was synthesized by Nanjing GenScript. ...
Embodiment 2
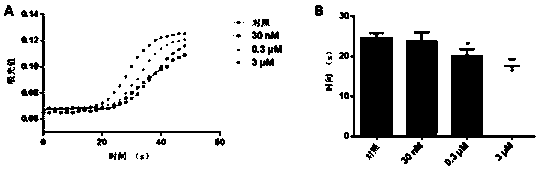
[0056] Example 2: Procoagulant polypeptide LGTX-F2 shortens recalcification and APTT time
[0057] APTT experimental method:
[0058] Equilibrate the APTT reagent to room temperature, gently invert the APTT reagent, and at the same time, place the CaCl2 solution in a water bath or a constant temperature incubator to preheat to 37°C. The final concentrations of the incubated samples were 0nM, 30nM, 0.3 μM, 3μM.
[0059] APTT test method
[0060] Table 3-1 The method of APTT experiment
[0061]
[0062] Plasma recalcification test method:
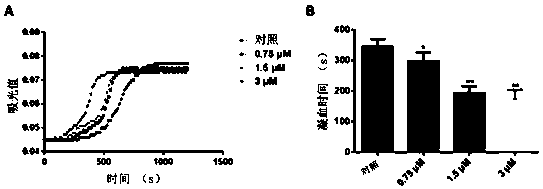
[0063] Combine 20 μL of normal human plasma (plus 3% sodium citrate for anticoagulation) with 3 μL of samples (incubate samples with final concentration of 0 μM, 0.75 μM, 1.5 μM, 3 μM). Mix in Hepes buffer (150 mM NaCl, pH 7.5) to a final volume of 70 μL. Then incubate for 10 min in a constant temperature incubator at 37°C. After incubation, add 50 μL of CaCl preheated at 37°C 2 solution (25 mM) to initiate the reaction. The enzyma...
Embodiment 3
[0065] Example 3: Procoagulant polypeptide LGTX-F2 shortens tail bleeding time
[0066] Experimental method of mouse tail bleeding model:
[0067] Take 20-25g Kunming mice and divide them into negative control group (saline), dosing group (0.625mg / kg, 1.25mg / kg, 2.5mg / kg) and positive control group (5mg / kg EACA), each group 6, randomly assigned, half male and half male. 15 minutes before tail clipping, 100 μl saline, 100 μl sample (0.625 mg / kg, 1.25 mg / kg, 2.5 mg / kg) and 100 μl EACA (5 mg / kg) were injected into tail vein respectively. After adding the medicine, cut the tail 7mm with a razor blade and immediately place it in a 37-degree constant temperature saline solution. The timing starts when the tail bleeds, and the timing stops when the bleeding stops. If bleeding occurs again within 120 seconds, continue timing, and so on. Repeat until the bleeding stops. At the end of the experiment, only the cumulative total time of bleeding was recorded. Statistical analysis of exp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com