Preparation method of clinical-grade human umbilical cord mesenchymal stem cell complex factor and lyophilized powder for repairing
A technology of stem cells and compound factors, which is applied in the fields of biochemical equipment and methods, animal cells, lyophilization and transportation, etc., can solve the problem that the medium is expensive and difficult to realize the productization of human umbilical cord mesenchymal stem cells compound factors and lyophilized powder. and other issues, to achieve the effect of safety and effectiveness, saving manpower and reducing immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
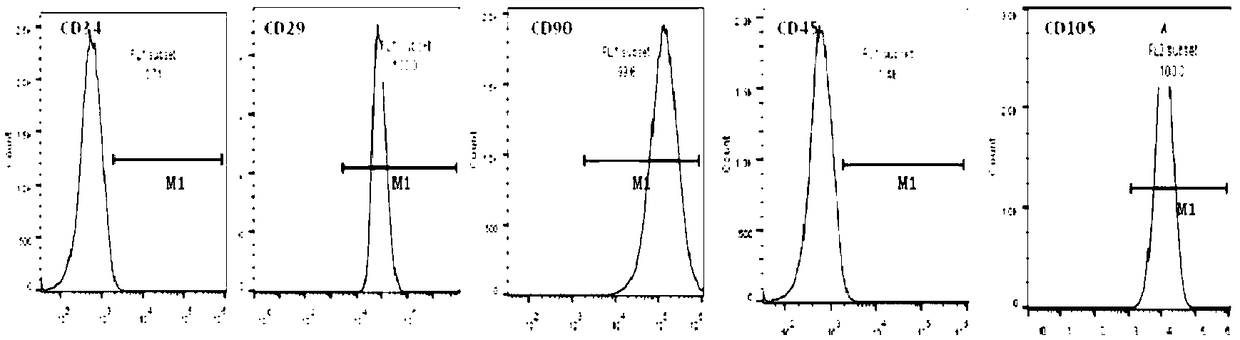
Image
Examples
Embodiment Construction
[0049] (1) Preparation of clinical grade stem cell complete medium
[0050] (1) Preparation of clinical-grade stem cell basal medium: mix the various amino acid solutions, vitamin solutions and glucose solutions used in clinical applications at a volume ratio of 69:1:20. After mixing, add 100 mg / L ascorbic acid. The following table shows the detailed composition of CRSCBM.
[0051] Table 1 Detailed composition table of CRSCBM
[0052]
[0053]
[0054] (2) Preparation of self-extracted platelet lysate: Collect machine-collected platelets that have been tested to meet clinical blood standards, mix and resuspend in sterile normal saline, centrifuge at 200g at room temperature for 10 minutes, and clean residual impurities. After cleaning, adjust the platelet concentration to 1×10 9 / ml, and place it at -80°C overnight. The next day, take it out and place it in a 37°C water bath to re-thaw, and repeat the freeze-thaw operation three times to make it fully lysed and release growth factor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com