Oral rehydration salt effervescent tablet and preparation method thereof
A technology of effervescent tablets and rehydration salts, applied in the field of oral rehydration salts, which can solve the problems of pollution, large volume, and insoluble auxiliary materials in water, etc., and achieve the effect of convenient use and clear appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] An oral rehydration salt effervescent tablet, the components of the oral rehydration salt effervescent tablet and the percentages of each component accounting for the total weight are: sodium chloride 5%, potassium chloride 2%, citrate 3%, anhydrous Glucose 30%, sodium bicarbonate 2%, pregelatinized starch 6%, Tween 800.2% and aspartame 0.1%.
Embodiment 2
[0038]An oral rehydration salt effervescent tablet, the components of the oral rehydration salt effervescent tablet and the percentages of each component accounting for the total weight are: sodium chloride 7.5%, potassium chloride 4%, citrate 4%, anhydrous Glucose 40%, sodium bicarbonate 6%, pregelatinized starch 9%, Tween 800.3% and aspartame 0.2%.
Embodiment 3
[0040] An oral rehydration salt effervescent tablet, the components of the oral rehydration salt effervescent tablet and the percentages of each component accounting for the total weight are: sodium chloride 10%, potassium chloride 6%, citrate 5%, anhydrous Glucose 50%, sodium bicarbonate 10%, pregelatinized starch 12%, Tween 800.4% and aspartame 0.3%.
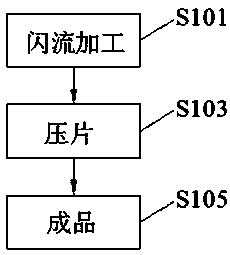
[0041] like figure 1 As shown, according to the embodiment of the present invention, a preparation method of oral rehydration salt effervescent tablet is also provided.
[0042] A preparation method for oral rehydration salt effervescent tablet, comprising the following steps:
[0043] Step S101, flash flow processing: mix the sodium bicarbonate, pregelatinized starch, Tween 80 and aspartame in the mixer, place the flash flow processing in the Econo Floss machine at a speed of 3600 rom, and form floc and mince;
[0044] Step S103, tablet compression: pass the main ingredients of the prescription amount through an 80-mesh si...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com