Aluminum salt adsorbent and application thereof in extraction of lithium in salt lake brine
A salt lake brine and adsorbent technology, applied in other chemical processes, process efficiency improvement, chemical instruments and methods, etc., can solve the problems of large dissolution loss rate, poor permeability and fluidity, etc., to reduce pollution and reduce impact. , the effect of reducing the dissolution loss rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
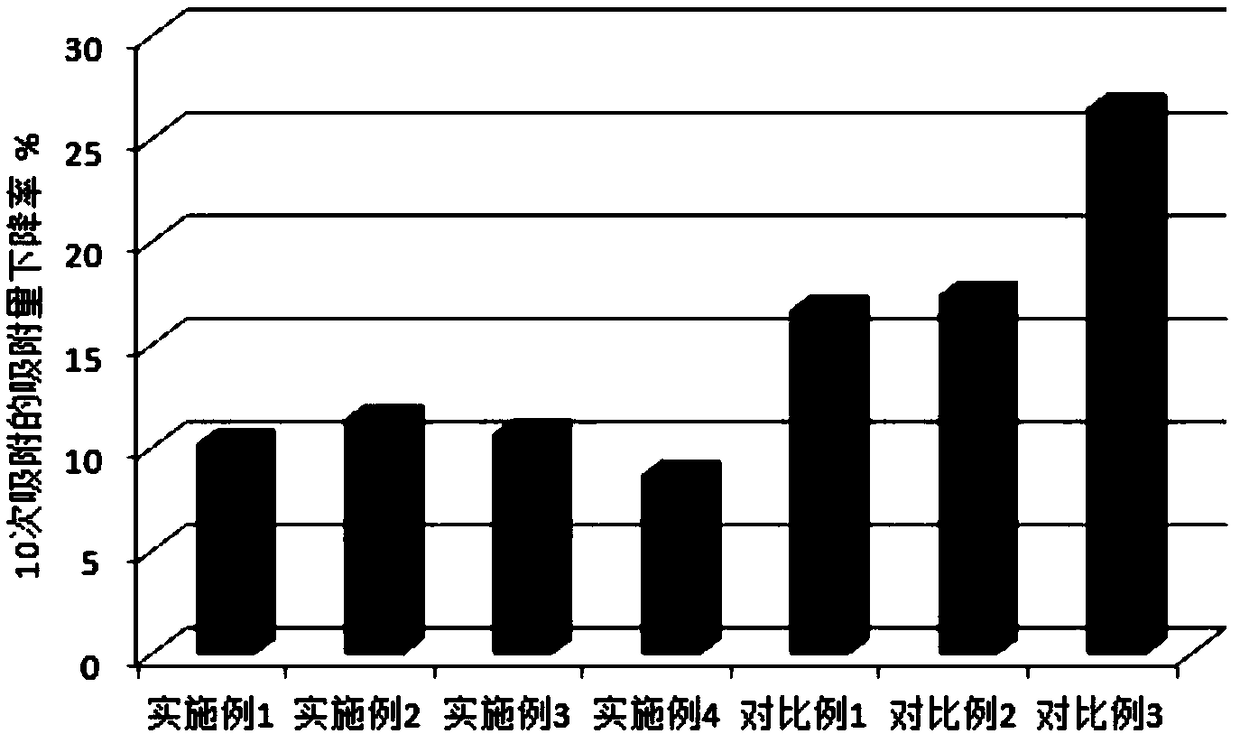
Embodiment 1
[0026] Preparation method of aluminum salt adsorbent:
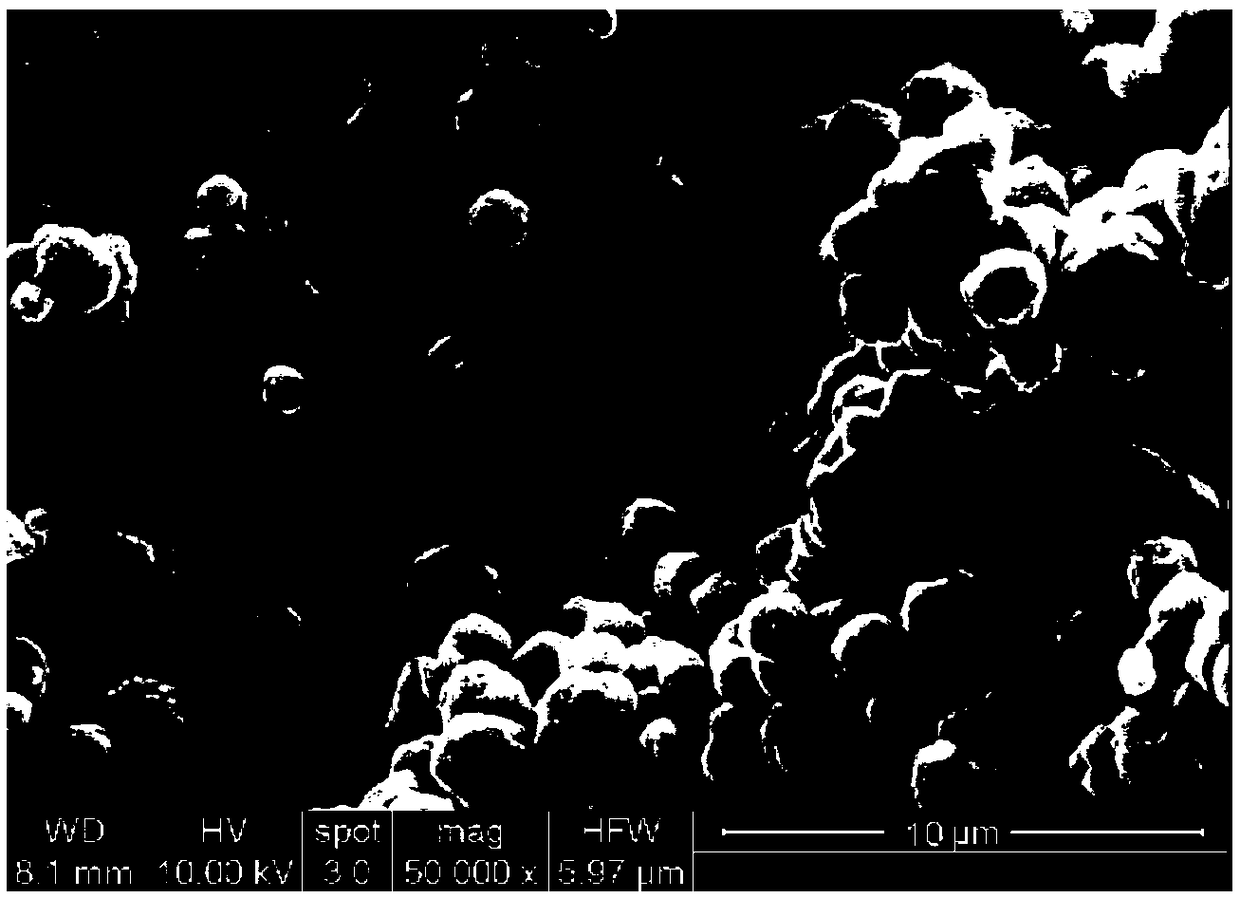
[0027] Step 1, the preparation of aluminum hydroxide: prepare an aqueous solution containing 2mol / L aluminum nitrate nonahydrate and 1mol / L lithium chloride, add urea for reaction, the molar ratio of urea to aluminum nitrate is 8:1, and the reaction time is 2h , the reaction temperature is 20°C. After the reaction, the obtained gel is centrifuged, washed with deionized water, and then centrifuged to obtain a lithium-intercalated aluminum hydroxide gel;
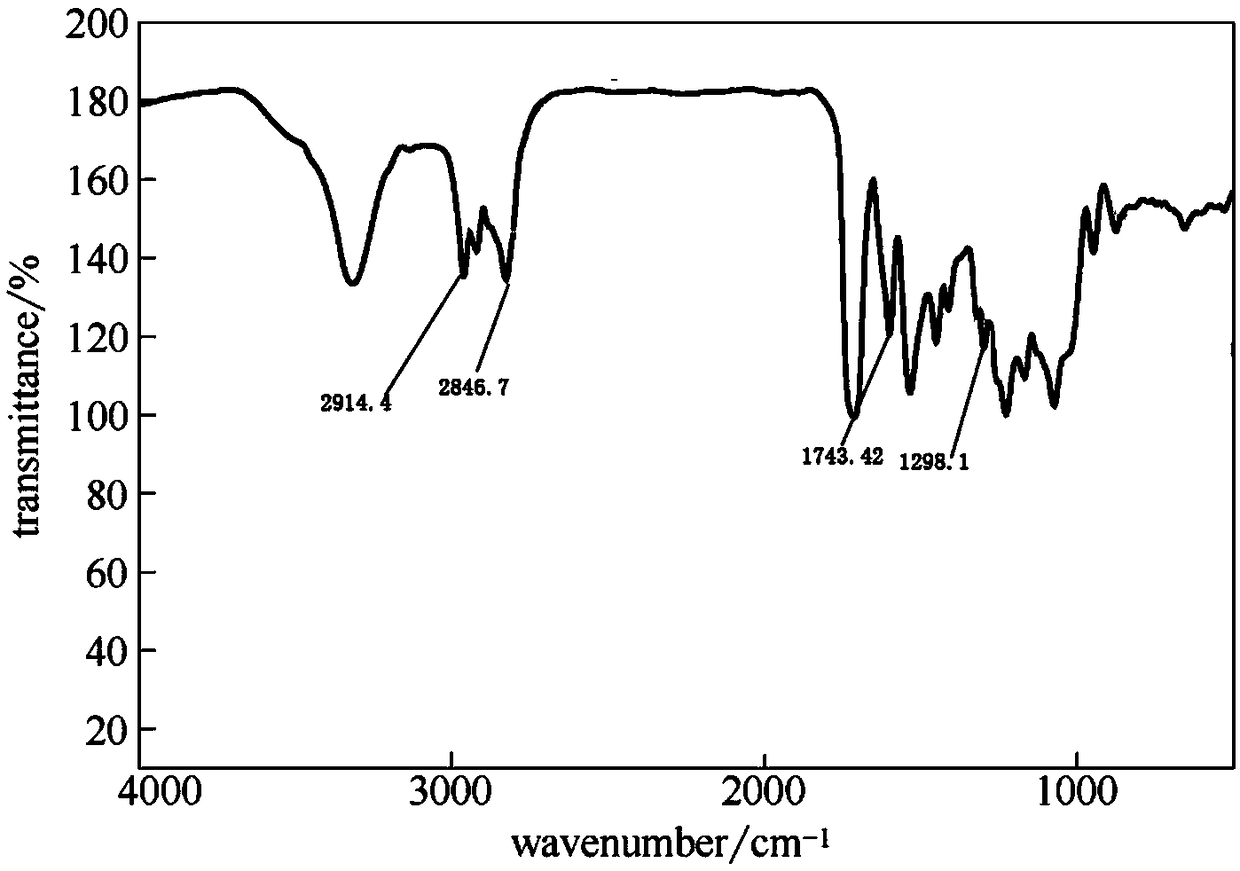
[0028] Step 2, modification of aluminum hydroxide: mix lithium-intercalated aluminum hydroxide gel with ethanol aqueous solution, and then add dropwise an ethanol solution of 5wt% γ-(methacryloyloxy)propyltrimethoxysilane for reaction , the weight ratio of lithium-intercalated aluminum hydroxide gel, ethanol aqueous solution, and ethanol solution of silane coupling agent is 1:8:5, the reaction time is 2 hours, and the reaction temperature is 25°C. After the reaction is co...
Embodiment 2
[0031] Preparation method of aluminum salt adsorbent:
[0032] Step 1, preparation of aluminum hydroxide: prepare an aqueous solution containing 5mol / L aluminum nitrate nonahydrate and 2mol / L lithium chloride, add urea for reaction, the molar ratio of urea to aluminum nitrate is 12:1, and the reaction time is 4h , the reaction temperature is 40°C. After the reaction, the obtained gel is centrifuged, washed with deionized water, and then centrifuged to obtain a lithium-intercalated aluminum hydroxide gel;
[0033] Step 2, modification of aluminum hydroxide: mix lithium-intercalated aluminum hydroxide gel with ethanol aqueous solution, and then dropwise add 10wt% ethanol solution of γ-(methacryloyloxy)propyltrimethoxysilane to react , the weight ratio of lithium-intercalated aluminum hydroxide gel, ethanol aqueous solution, and ethanol solution of silane coupling agent is 1: 12: 10, the reaction time is 3 hours, and the reaction temperature is 35 ° C. After the reaction is compl...
Embodiment 3
[0036] Preparation method of aluminum salt adsorbent:
[0037] Step 1, preparation of aluminum hydroxide: prepare an aqueous solution containing 4mol / L aluminum nitrate nonahydrate and 2mol / L lithium chloride, add urea for reaction, the molar ratio of urea to aluminum nitrate is 10:1, and the reaction time is 3h , the reaction temperature is 30°C. After the reaction, the obtained gel is centrifuged, washed with deionized water, and then centrifuged to obtain a lithium-intercalated aluminum hydroxide gel;
[0038] Step 2, modification of aluminum hydroxide: mix lithium-intercalated aluminum hydroxide gel with ethanol aqueous solution, and then add dropwise an ethanol solution of 5wt% γ-(methacryloyloxy)propyltrimethoxysilane for reaction , the weight ratio of lithium-intercalated aluminum hydroxide gel, ethanol aqueous solution, and ethanol solution of silane coupling agent is 1:10:7, the reaction time is 2 hours, and the reaction temperature is 30°C. After the reaction, the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com