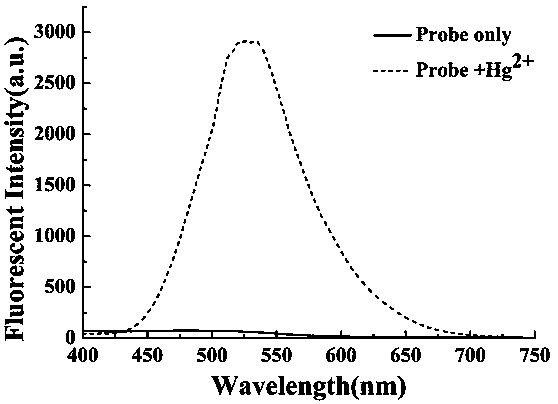
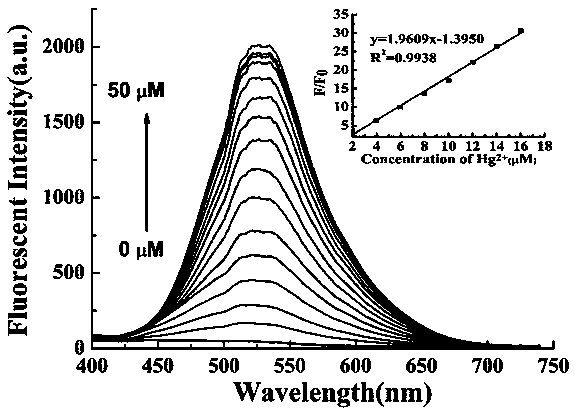
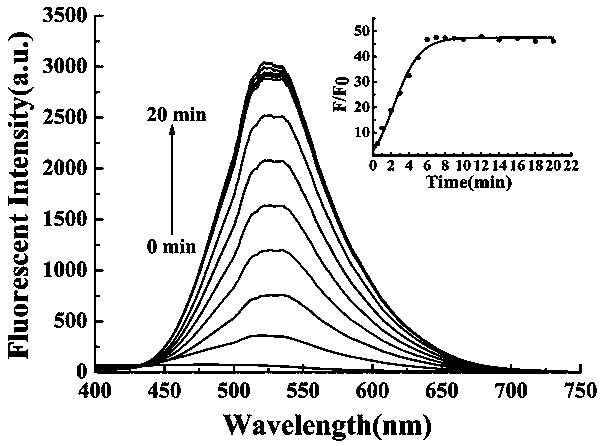
Preparation method and application of turn-on type mercury ion fluorescence probe
A fluorescent probe, mercury ion technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of weak anti-interference ability, long response time, poor water solubility, etc., to achieve high selectivity and sensitivity, Sensitive, easy-to-synthesize effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment one: the synthesis of compound 3
[0032] Add (5.821g, 0.0625mol) 4-picoline, (6.100g, 0.05mol) 4-hydroxybenzaldehyde and 10mL acetic anhydride solution to a 25mL three-neck flask equipped with a magnetic stirrer, and heat to reflux for 12h. After the reaction, add 500mL of ice water and stir for 1h to produce a precipitate, which is washed with water and recrystallized with ethanol, then refluxed in a solution containing 90mL of ethanol and (3.780g, 0.0675mol) potassium hydroxide for 1.5h, and then added acetic acid to Precipitation occurred, washed with water and dried to obtain compound 4-(4-hydroxystyryl)pyridine, namely compound 3, 7.232g, yield 73.4%. 1 HNMR (400MHz, DMSO, 25℃, TMS) δ9.83 (broad, -OH), 8.50 (d, J = 5.6, 2H), 7.51 (d, J = 6.4, 4H), 7.45 (d, J = 16.4 ,1H),7.02(d,J=16.4,1H),6.82(d,J=8.4,2H). 13 CNMR (100MHz, CDCl 3 , 25℃, TMS) δ158.2, 149.9, 144.8, 133.0, 128.7, 127.2, 122.4, 120.5, 115.7.
Embodiment 2
[0033] Embodiment two: the synthesis of compound 3
[0034] Add (5.588g, 0.06mol) 4-picoline, (6.100g, 0.05mol) 4-hydroxybenzaldehyde and 10mL acetic anhydride solution to a 25mL three-neck flask equipped with a magnetic stirrer, and heat to reflux for 10h. After the reaction, add 500mL of ice water, stir for 0.8h, a precipitate occurs, wash the precipitate with water and recrystallize with ethanol, then reflux in a solution containing 90mL of ethanol, (3.528g, 0.063mol) potassium hydroxide for 1.4h, add acetic acid When precipitation occurs, it is washed with water and dried to obtain compound 4-(4-hydroxystyryl)pyridine, namely compound 3, 6.895 g, yield 70.0%. 1 HNMR (400MHz, DMSO, 25°C, TMS) δ9.82 (broad, -OH), 8.51 (d, J = 5.6, 2H), 7.50 (d, J = 6.4, 4H), 7.46 (d, J = 16.4 ,1H),7.02(d,J=16.4,1H),6.82(d,J=8.4,2H). 13 CNMR (100MHz, CDCl 3 , 25℃, TMS) δ158.0, 149.9, 144.7, 133.0, 128.7, 127.2, 122.3, 120.5, 115.4.
Embodiment 3
[0035] Embodiment three: the synthesis of compound 3
[0036] Add (7.264g, 0.078mol) 4-picoline, (6.100g, 0.05mol) 4-hydroxybenzaldehyde and 10mL acetic anhydride solution successively into a 25mL three-neck flask equipped with a magnetic stirrer, and heat to reflux for 11h. After the reaction, add 500mL of ice water, stir for 1.2h, a precipitate occurs, wash the precipitate with water and recrystallize with ethanol, then reflux in a solution containing 90mL of ethanol, (4.032g, 0.072mol) potassium hydroxide for 1.6h, add acetic acid When precipitation occurs, it is washed with water and dried to obtain compound 4-(4-hydroxystyryl)pyridine, namely compound 3, 6.402 g, yield 65.0%. 1 HNMR (400MHz, DMSO, 25℃, TMS) δ9.83 (broad, -OH), 8.49 (d, J = 5.6, 2H), 7.52 (d, J = 6.4, 4H), 7.47 (d, J = 16.4 ,1H),7.02(d,J=16.4,1H),6.80(d,J=8.4,2H). 13 CNMR (100MHz, CDCl 3 , 25℃, TMS) δ158.1, 149.9, 144.7, 133.1, 128.7, 127.1, 122.4, 120.5, 115.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com