Polymer luminescent material, preparation method and use thereof
A technology of luminescent materials and polymers, applied in the direction of luminescent materials, chemical instruments and methods, etc., to achieve the effect of improving photophysical properties, high fluorescence quantum efficiency and device efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
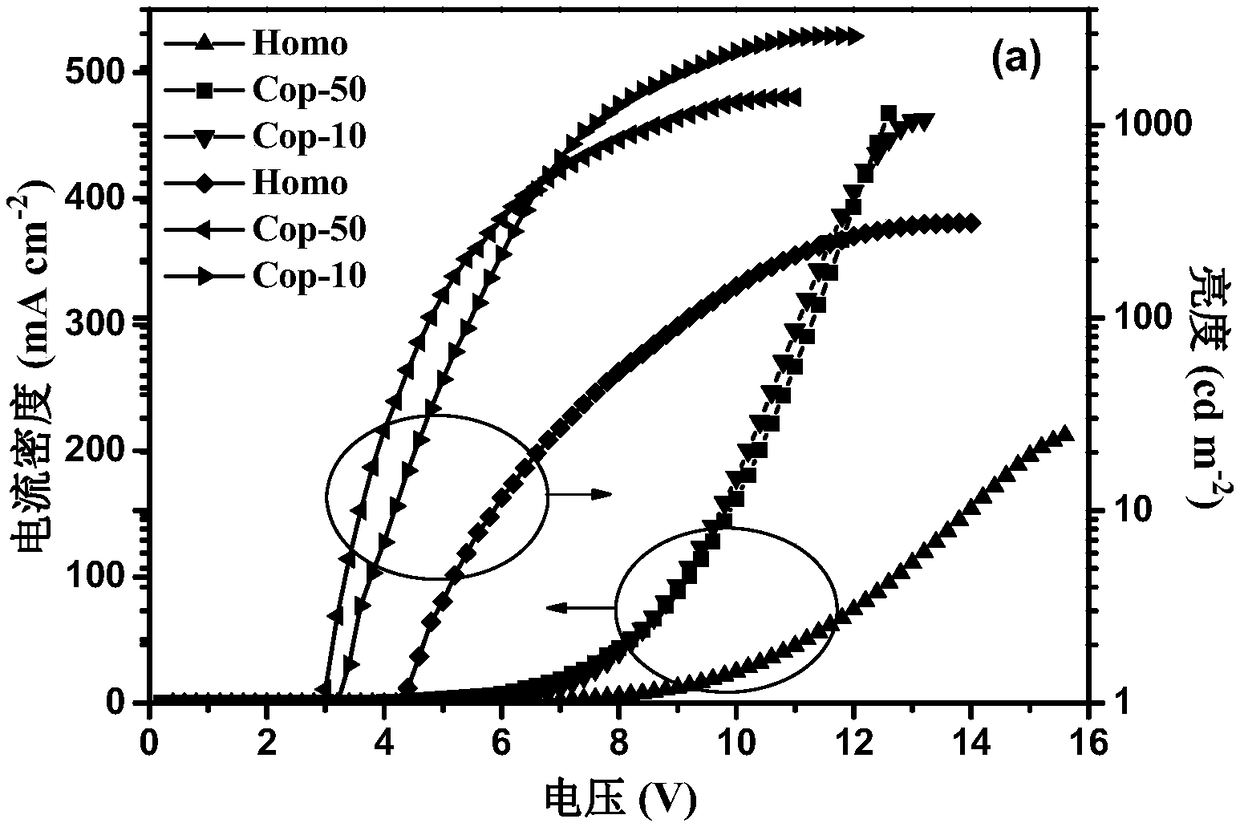
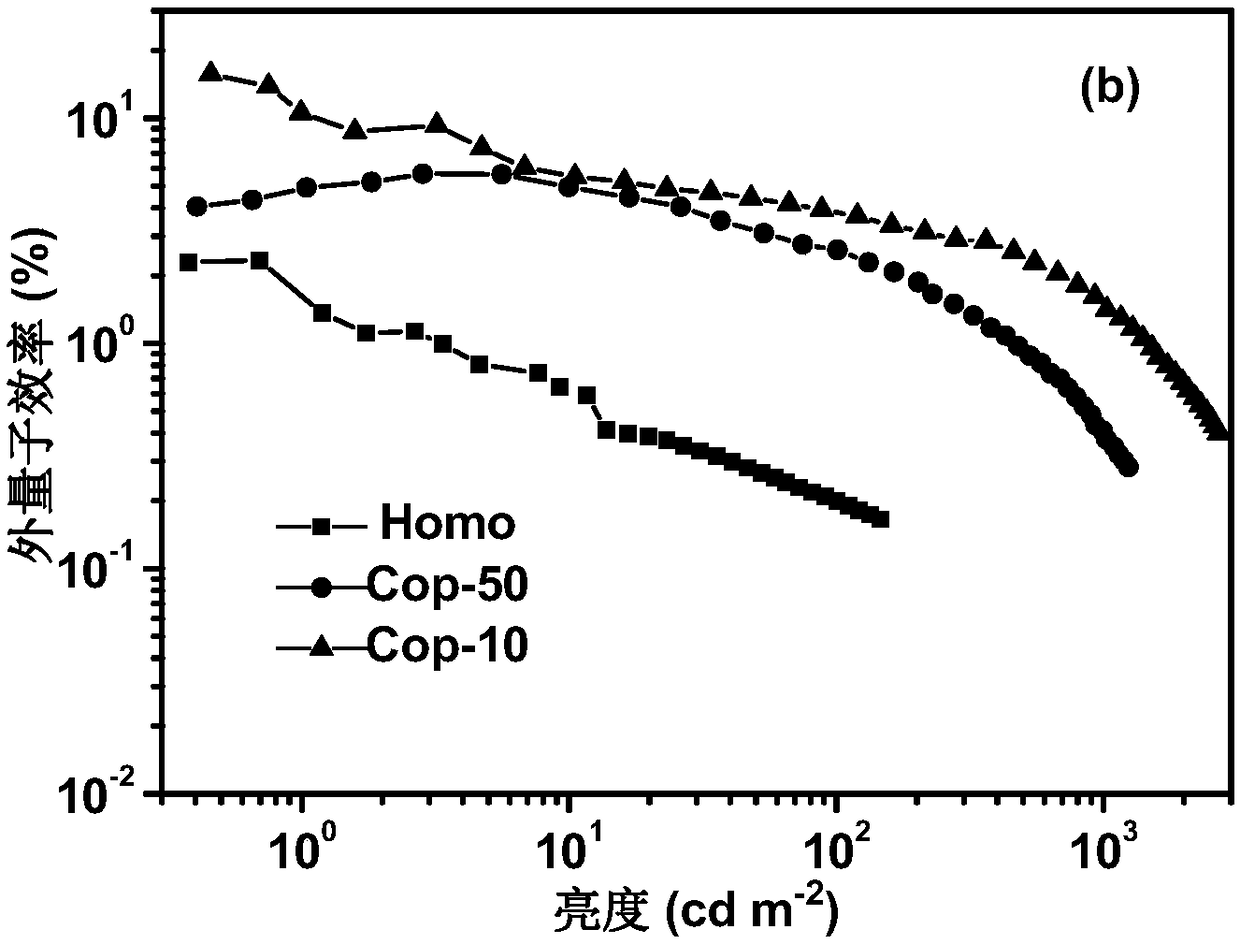
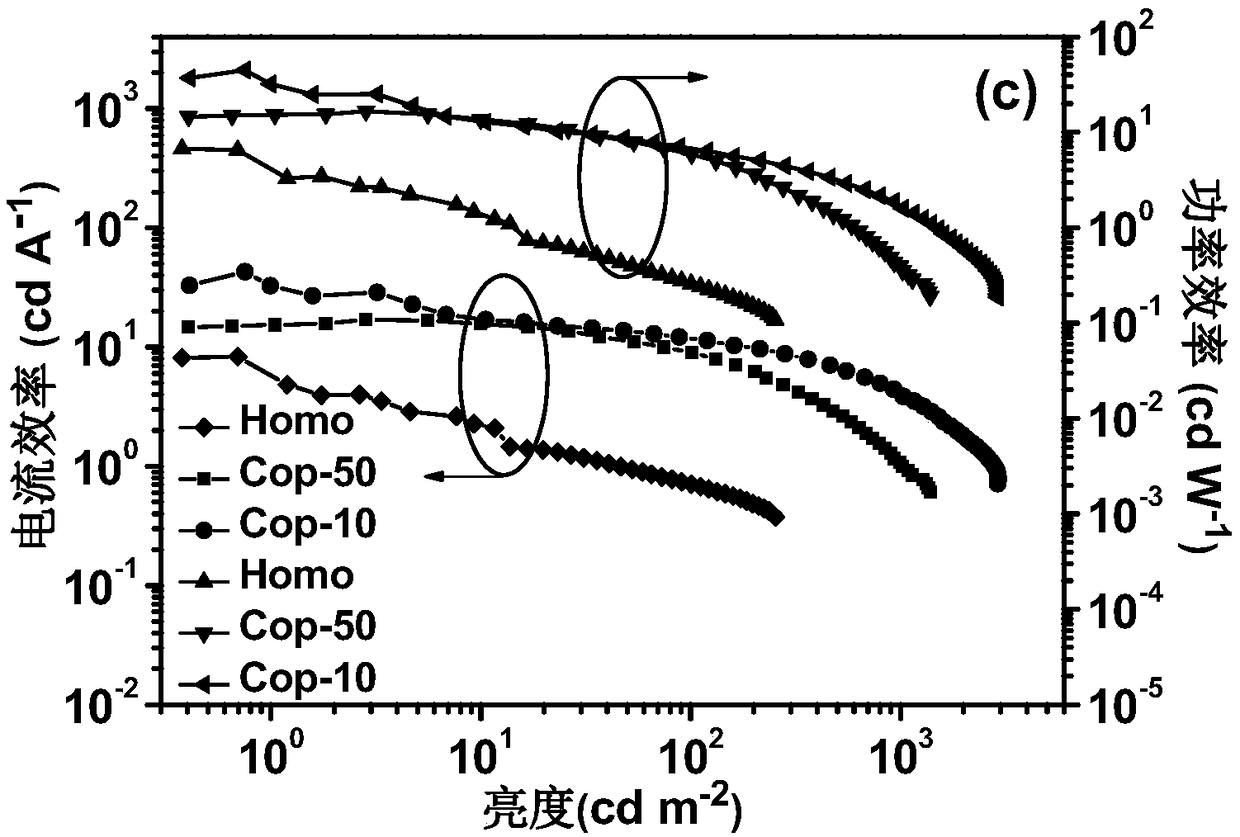
Image
Examples
preparation example 1
[0082] Preparation Example 1: Synthesis of 2-(2-ethylhexyl)-8-phenothiazine dibenzothiophene sulfone
[0083] 1) Synthesis of 2-bromodibenzothiophene
[0084]
[0085] Under ice-water bath conditions, dibenzothiophene (0.1 mol) was dissolved in 100 ml of anhydrous CHCl in the reactor 3 , then use 20ml of anhydrous CHCl 3 Dissolve 5.4ml of liquid bromine in a constant pressure funnel and add it dropwise to the reactor. React at room temperature for three days. Add 30ml saturated NaHSO 3 solution, stirred until the solution was white, and the organic layer was extracted. Then the reaction solution was poured into a separatory funnel, extracted 3 times with dichloromethane, and washed 3 times with saturated NaCl aqueous solution. The organic layer was dried with anhydrous sodium sulfate, filtered, spin-dried, and recrystallized from ethanol to obtain 25.2 g of a white solid with a yield of 96%.
[0086] 2) Synthesis of 2-(2-ethylhexanoyl)-8-bromo-dibenzothiophene
[0...
preparation example 2
[0095] Preparation Example 2: Synthesis of N-heptylcarbazole
[0096] Carbazole (50mmol) was dissolved in N,N-dimethylformamide DMF (100mL), and 60wt% NaH (75mmol) dispersed in kerosene was slowly added at 0°C and stirred for 3h. After the temperature naturally rose to room temperature, a solution of 1-bromo-n-heptane (55 mmol) in DMF (20 mL) was slowly dropped into the reaction system, and the reaction was continued for 12 h. After the reaction was completed, water was added to quench the reaction, and the organic phase was extracted with dichlorohexane. After spin-drying, the mixture of dichloromethane and n-hexane with a volume ratio of 1:5 was used as the eluent for column chromatography to obtain 11.8 g of a yellow solid with a yield of 89%. 1 H NMR (400MHz, Acetone-d6): δ8.11 (d, J = 7.8Hz, 2H), 7.50 (d, J = 8.2Hz, 2H), 7.48-7.36 (m, 2H), 7.18 (t, J =7.4Hz, 2H), 4.34 (t, J = 7.2Hz, 2H), 1.37-1.13 (m, 10H), 0.83 (dt, J = 13.7, 6.8Hz, 3H).
[0097]
Embodiment 1
[0099] (1) Synthesis of 2-(2-ethylhexyl)-8-(3,7-dibromophenothiazine) dibenzothiophene sulfone (terminal brominated monomer A)
[0100]
[0101]2-(2-Ethylhexyl)-8-phenothiazinedibenzothiophene sulfone (4 mmol) was dissolved in 50 mL of dry dichloromethane. In an ice-water bath and protected from light, a solution of NBS (8.4 mmol) in dry dichloromethane was slowly added dropwise thereto. After stirring at room temperature for 20 h, the reaction liquid was added into water to terminate the reaction. Afterwards, the organic phase was extracted with dichloromethane, dried over magnesium sulfate, and spin-dried. Using a mixture of n-hexane and dichloromethane (the volume ratio of the two is 1:1) as the eluent for column chromatography to obtain 2.5 g of light yellow solid with a yield of 91%. 1 H NMR (400MHz, DMSO-d6): δ8.32(s, 1H), 8.22(d, J=8.1Hz, 1H), 8.10(s, 1H), 8.41-7.68(m, 8H), 7.92(d ,J=7.9Hz,1H),7.65(d,J=8.1Hz,1H),7.48(d,J=7.9Hz,1H),7.43(s,2H),7.18(d,J=8.8Hz,2H ),6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com