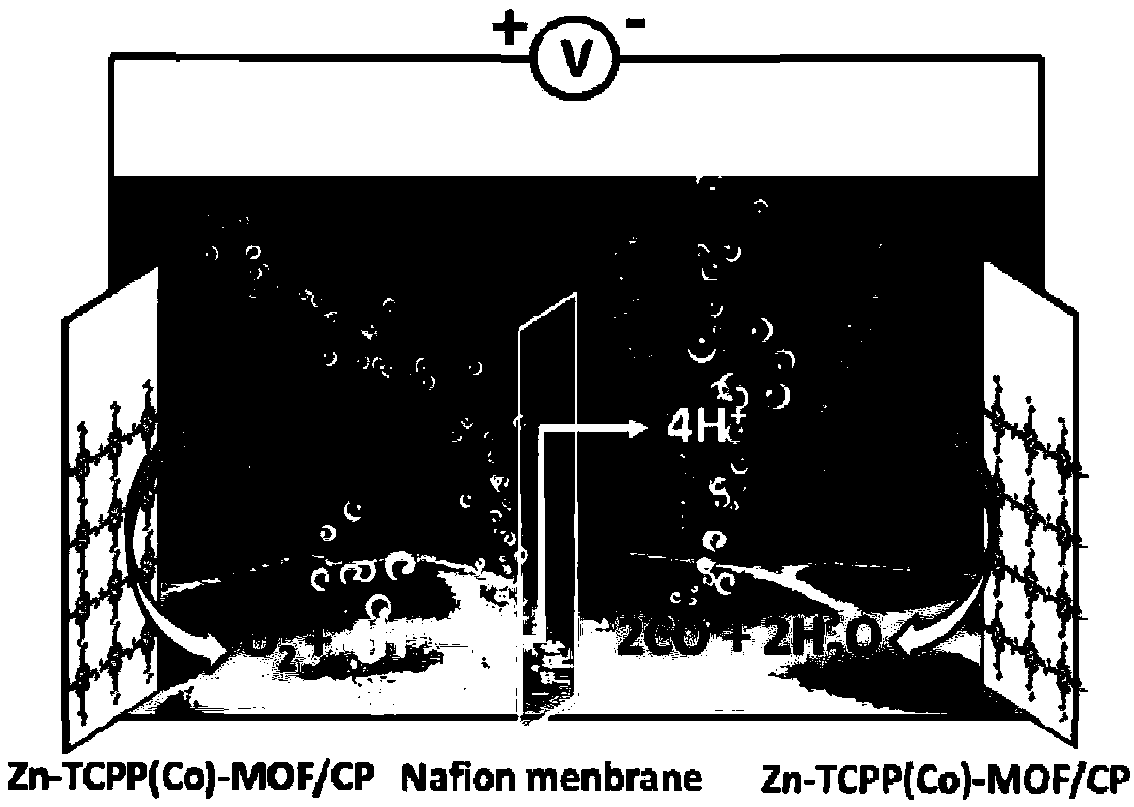
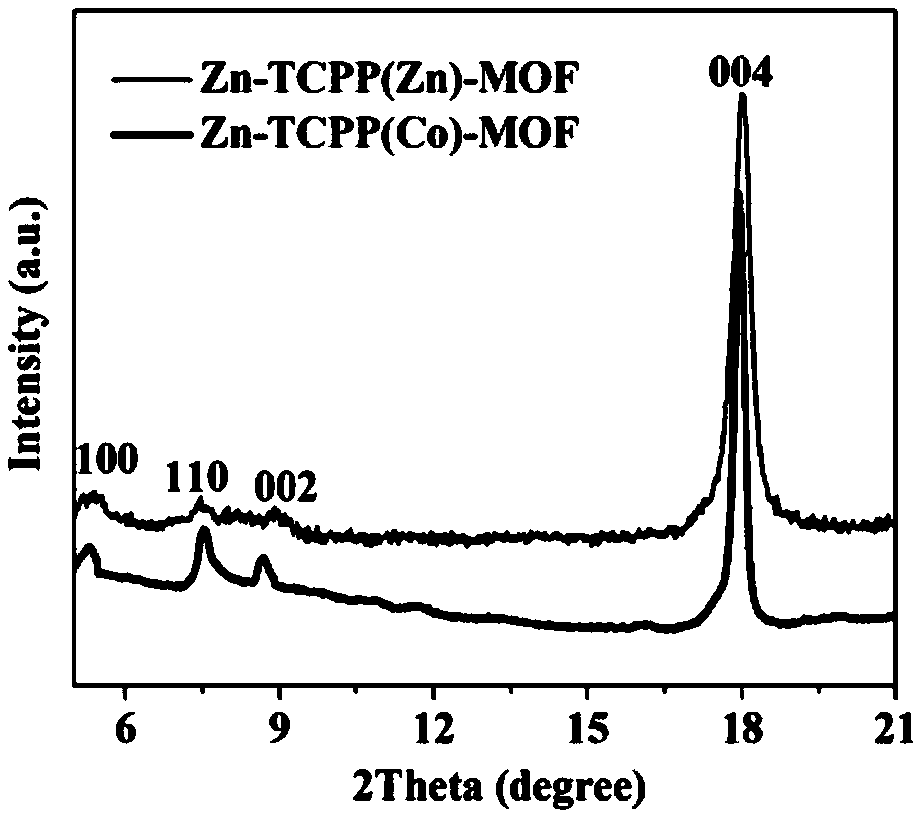
Preparation method for electrocatalytic carbon dioxide reduction electrode, and application thereof
A technology of carbon dioxide and electrocatalysis, applied in the direction of electrodes, electrolysis process, electrolysis components, etc., can solve environmental pollution and other problems, and achieve the effect of excellent catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 MOF catalyst
[0032] (1) Preparation of Zn-TCPP(Co)-MOF
[0033] Add cobalt nitrate hexahydrate (2.9mg, 10μmol), TCPP (7.9mg, 10μmol) and DMF (5mL) into a 10mL two-necked round bottom flask, sonicate for 30min and put it in an oil bath for reflux reaction under nitrogen protection for 3h. After the reaction was completed, it was naturally cooled to room temperature, and then 1.5 mL of absolute ethanol and zinc nitrate hexahydrate (5.9 mg, 20 μmol) were added to the flask, heated to 80° C. to continue the reaction for 24 h, and then naturally cooled to room temperature. The obtained red crystals were separated by a high-speed centrifuge, washed three times with DMF and ethanol, and dried in vacuum at 25°C for 12 hours to obtain a red solid Zn-TCPP(Co)-MOF.
[0034] (2) Preparation of Zn-TCPP(Zn)-MOF
[0035] DMF (6mL) and absolute ethanol (2mL) were added to a 20mL round bottom flask to obtain a mixed solvent, zinc nitrate (17.8mg, 60μm...
Embodiment 2
[0044] Example 2 Preparation of Zn-TCPP(Co)-MOF / CP electrode
[0045] Before preparing the Zn-TCPP(Co)-MOF / CP electrode, carbon paper (CP, 1×2.5cm 2 ) were washed with concentrated nitric acid, ethanol and ultrapure water to remove organic matter and metal impurities on the surface of carbon paper. Get 2.5mgZn-TCPP(Co)-MOF and add the mixed solution (V EtOH :V PEI :V Nafion =1000:1:2), sonicate for 30 minutes to obtain a uniform dispersion. Take 0.1mL of the dispersion and evenly drop it on the carbon paper (the active area is 1cm 2 ), and then dried in air overnight to obtain the Zn-TCPP(Co)-MOF / CP electrode. Zn-TCPP(Zn)-MOF / CP electrodes can be prepared by the same method.
Embodiment 3
[0046] Example 3 Test of electrochemical properties of Zn-TCPP(Co)-MOF / CP electrode
[0047] Cyclic voltammetry and electrochemical stability test of three-electrode system
[0048] In a closed electrolytic cell, a three-electrode system was used to test the half-reaction of electrochemical carbon dioxide reduction. The Zn-TCPP(Co)-MOF / CP electrode was used as the working electrode, the platinum wire electrode was used as the counter electrode, and Ag / AgCl (saturated KCl solution) The electrode is the reference electrode, 0.5M CsHCO 3 The aqueous solution (pH=8.6) is the electrolyte solution. First pass argon gas into the electrolyte for 0.5h to discharge the electrolytic cell and oxygen dissolved in the electrolyte, and then carry out cyclic voltammetry scanning (scanning rate 50mV / s) in nitrogen protection. Then, carbon dioxide was introduced into the electrolytic cell for 0.5h until the carbon dioxide was dissolved and saturated in the electrolyte (pH=7.3), and then a cyc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com