Positive active material for aqueous zinc ion secondary battery and aqueous zinc ion secondary battery
A cathode active material, zinc ion battery technology, applied in secondary batteries, battery electrodes, circuits, etc., can solve the problems of high charge density, low activity, difficulty in de-intercalation and migration, and achieve the effect of high working voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The preparation method of described lithium vanadyl phosphate is as follows:
[0043] Mixing citric acid, vanadium source compound and water for reaction;
[0044] adding an ammonium salt compound and a lithium salt compound to the above reaction product, mixing, evaporating to remove water, and obtaining a gel;
[0045] The gel is subjected to high-temperature calcination to obtain LiVOPO 4 ;
[0046] Specifically, the vanadium source compound is selected from vanadium pentoxide, and the molar ratio of the citric acid to the vanadium source compound is (2-8):1.
[0047] After vanadium pentoxide is completely dissolved, add ammonium phosphate compound and lithium salt compound to the above solution, mix, evaporate and remove water, and obtain a gel;
[0048] Wherein, the ammonium phosphate compound is selected from ammonium dihydrogen phosphate; the lithium salt compound is selected from lithium nitrate;
[0049] The evaporation temperature is 100°C-120°C, preferabl...
Embodiment 1
[0086] Accurately weigh 0.01mol of vanadium pentoxide and 0.04mol of citric acid, pour them into a beaker filled with distilled water in turn, heat to 60°C and keep stirring until it becomes a dark blue solution. Then slowly add 0.02mol ammonium dihydrogen phosphate and 0.02mol lithium nitrate respectively, and raise the temperature to 100-120°C, evaporate and remove the water until a gel is formed; then place the obtained gel in a 120°C air blast to dry In the box, the precursor can be obtained. Finally, after calcination at a high temperature of 500 ° C, LiVOPO is obtained 4 .
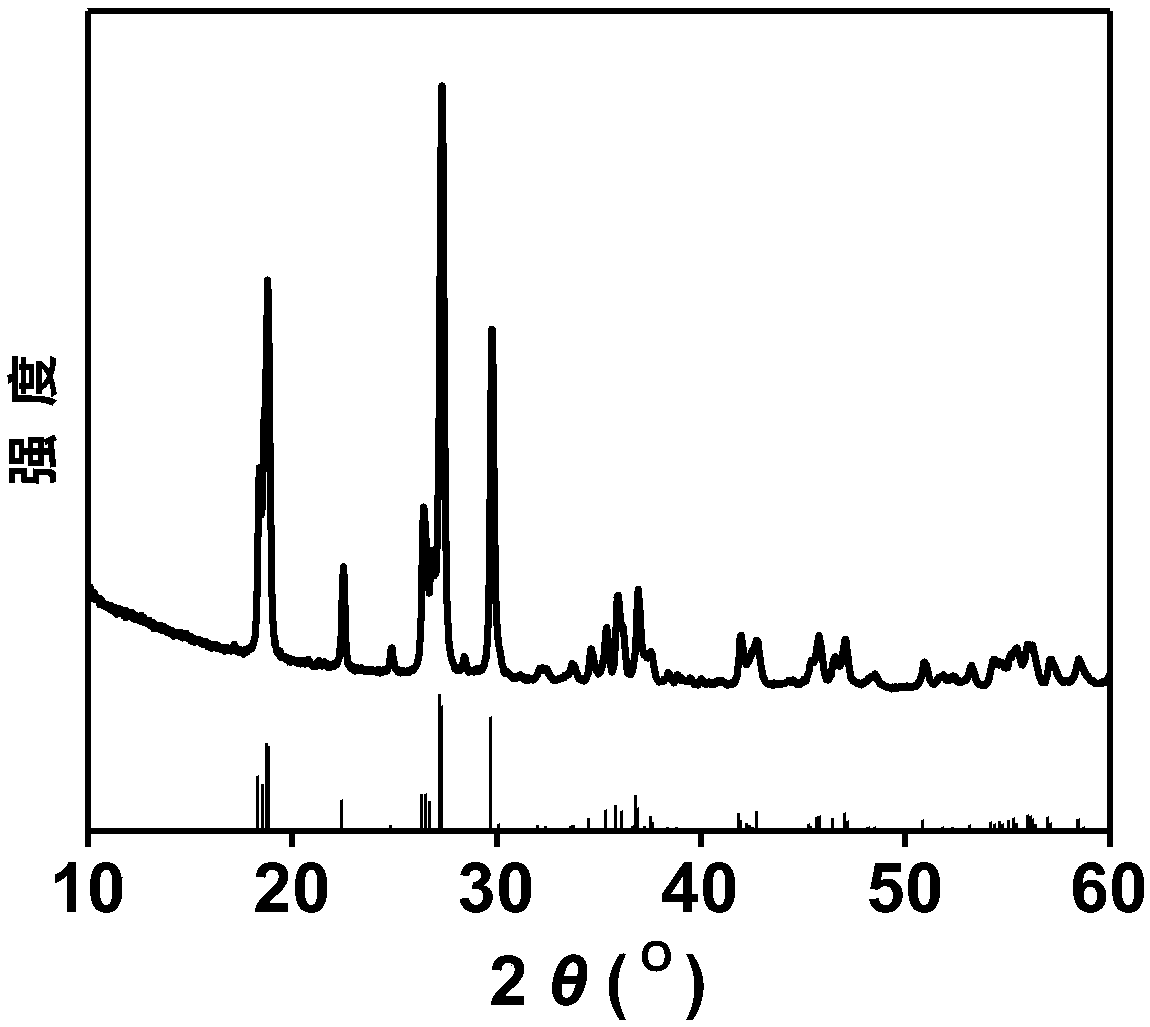
[0087] LiVOPO capable of deintercalating divalent zinc ions prepared by sol-gel method 4 The positive electrode material, its XRD diffraction pattern is as follows figure 1 shown. pass figure 2 It can be seen that it is composed of rod-shaped lithium vanadyl phosphate particles of different sizes and irregular cross-sections through interlacing to form a porous layered morphology. The material...
Embodiment 2
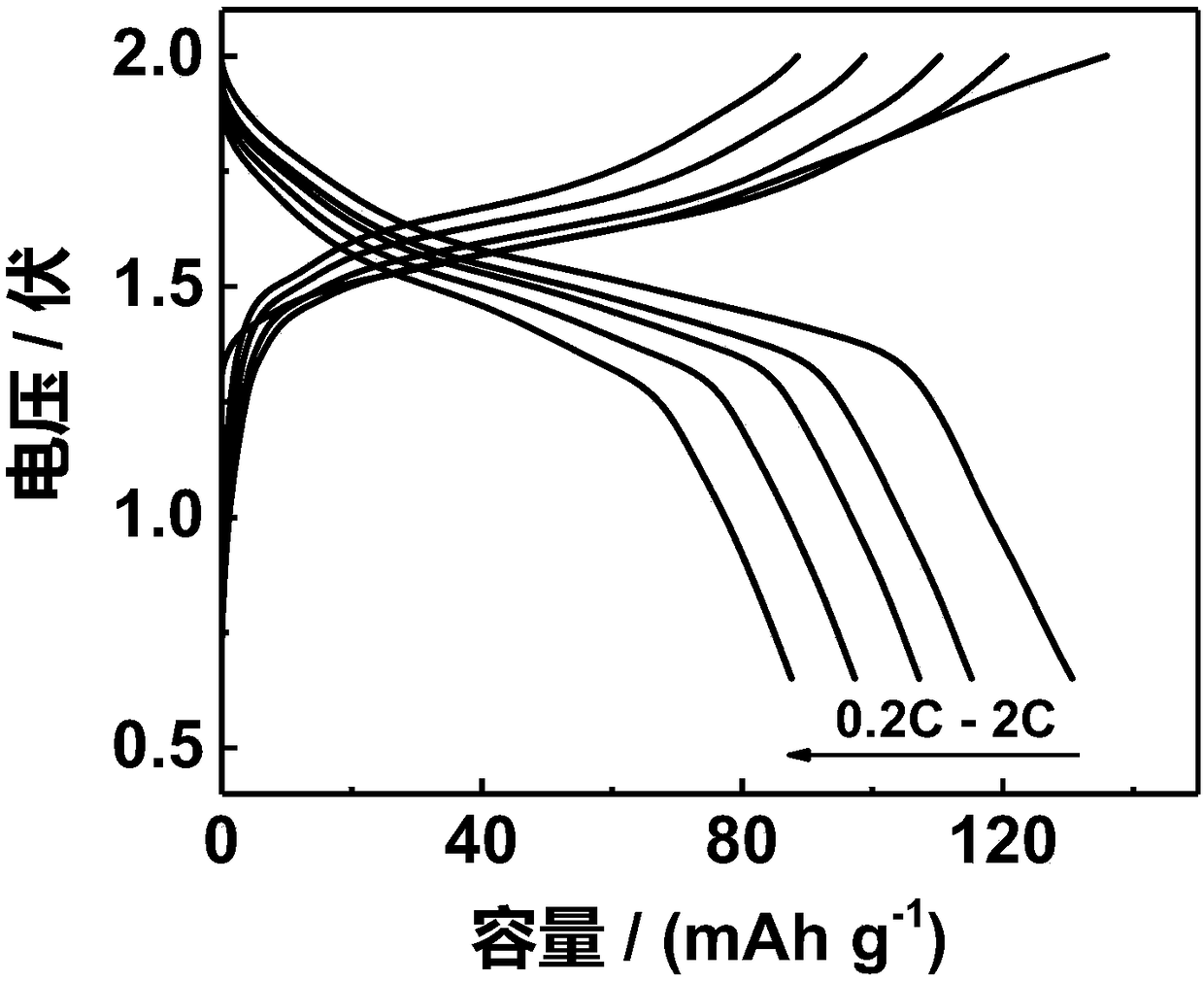
[0103]This example is basically the same as Example 1, except that the electrolyte is zinc perchlorate with a molar concentration of 7 mol / L, and a water-based zinc-ion battery is constructed by the method of Example 1. The assembled aqueous zinc-ion secondary battery was charged and discharged under constant current. However, the battery has a lower discharge capacity than that of Example 1, which is about 120mAh g at 0.2C after different rate cycle charge and discharge tests. -1 , while also exhibiting a poor Coulombic efficiency, below 95%. Illustrate that in this example, the addition of a large amount of Polyethylene Glycol (the raising of concentration just reduces the content of water, but the ratio of zinc perchlorate and Polyethylene Glycol remains unchanged) although it is beneficial to improve the voltage window of zinc ion electrolyte And promote the stability of the material, but it greatly reduces the ionic conductivity, is not conducive to the transport of ions...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cross section size | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com