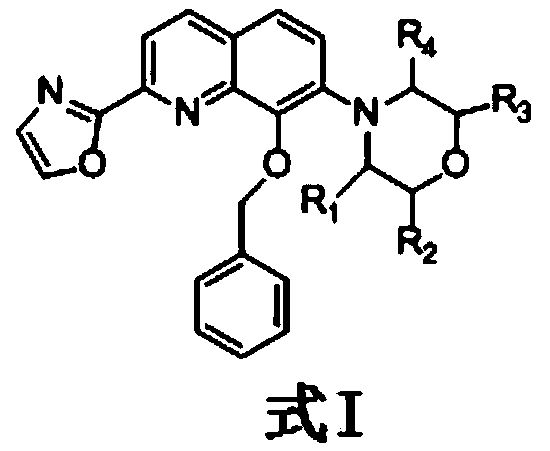
Compound having selective inhibitory activity against human fatty acid amide hydrolase and use thereof for treating pains
A fatty amide, inhibitory activity technology, used in organic chemistry, non-central analgesics, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Synthesis of 2-(8-(benzyloxy)-7-(morpholin-4-yl)quinolin-2-yl)oxazole
[0027] 1. Synthesis of 7-bromo-N-(2,2-dimethoxyethyl)-8-hydroxyquinoline-2-carboxamide:
[0028]
[0029] To a suspension of 7-bromo-8-hydroxyquinoline-2-carboxylic acid (compound 1) (77.74 g, 290 mmol) in ethyl acetate (200 mL) was added thionyl chloride (41.40 g, 348 mmol) at room temperature , and heated to reflux for 3 hours. The reaction mixture was cooled to room temperature, diluted with tetrahydrofuran (50 mL), and then 2,2-dimethoxyethane-1-amine (42.69 g, 406 mmol) and triethylamine (110 mL, 789 mmol) were added dropwise at room temperature solution in tetrahydrofuran (250 mL). The reaction mixture was then stirred at 40°C for 1 hour, cooled to room temperature, added with 300 mL of water and extracted with ethyl acetate. The organic layer was washed successively with 2mol / L hydrochloric acid, 10% potassium carbonate aqueous solution and water. The solvent was concentrate...
Embodiment 2
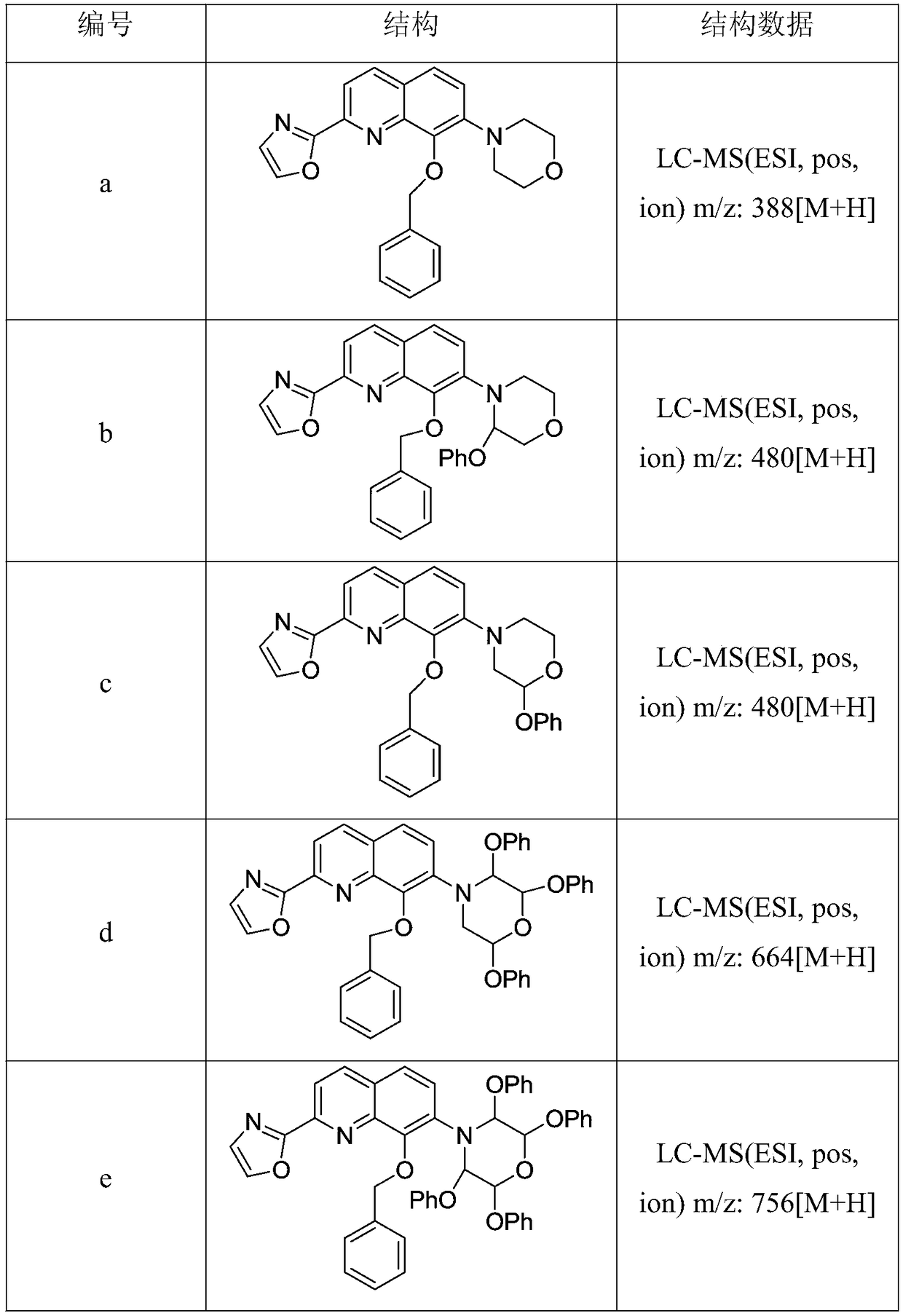
[0042] Example 2: Synthesis of 2-(8-(benzyloxy)-7-(2-phenoxy-morpholin-4-yl)quinolin-2-yl)oxazole
[0043]
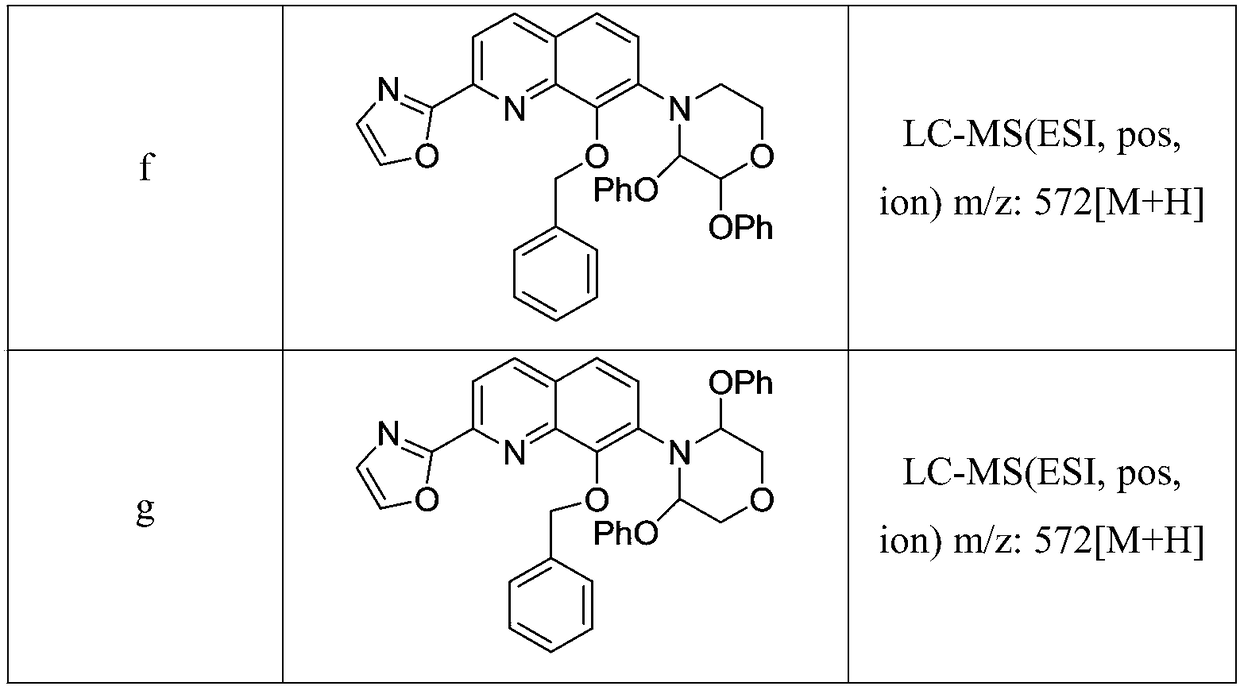
[0044] Palladium acetate (2.40 g), sodium p-toluenesulfonate (3.90 g), sodium tert-amylate (11.8 g) were added to a suspension of 2-phenoxy-morpholine (52 mmol) in toluene under an argon atmosphere. solution in toluene (37 mL), diadamantanebutylphosphine (7.70 g), and stirred at room temperature for 1 hour. In the reaction suspension, compound 5 (19.10 g, 50.10 mmol) and toluene (33 mL) were added under ice-water bath conditions, and then heated to reflux for 4 hours. After the reaction was completed, the reaction mixture was cooled to room temperature, and 2mol / L hydrochloric acid (37.5 mL) was added. The formed precipitate was collected by filtration to give 20.75 g of light brown crystals of 2-(8-(benzyloxy)-7-(2-phenoxy-morpholin-4-yl)quinolin-2-yl)oxazole, Yield 88%. LC-MS (ESI, pos, ion) m / z: 480[M+H].
Embodiment 3
[0045] Example 3: Synthesis of 2-(8-(benzyloxy)-7-(2,3-diphenoxy-morpholin-4-yl)quinolin-2-yl)oxazole
[0046]
[0047] Under an argon atmosphere, to a suspension of 2,3-diphenoxy-morpholine (52 mmol) in toluene was added palladium acetate (2.40 g), sodium p-toluenesulfonate (3.90 g), sodium tert-amylate ( 11.8 g) in toluene (37 mL), diadamantanebutylphosphine (7.70 g), and stirred at room temperature for 1 hour. In the reaction suspension, compound 5 (19.10 g, 50.10 mmol) and toluene (33 mL) were added under ice-water bath conditions, and then heated to reflux for 4 hours. After the reaction was completed, the reaction mixture was cooled to room temperature, and 2mol / L hydrochloric acid (37.5 mL) was added. The formed precipitate was collected by filtration to give 22.91 g of light brown crystals of 2-(8-(benzyloxy)-7-(2,3-diphenoxy-morpholin-4-yl)quinolin-2-yl) Oxazole, 80% yield. LC-MS (ESI, pos, ion) m / z: 572[M+H].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com