Duck reovirus causing duck spleen necrosis as well as inactivated vaccine and application thereof
A technology of reovirus and inactivated vaccine is applied in the field of duck reovirus and its inactivated vaccine, which can solve the economic loss of the breeding duck breeding industry, the egg production of breeding ducks and the weight loss of meat ducks, and the reduction of meat ducks. The problem of reducing the pass rate of slaughtering and other problems has achieved the effect of preventing and controlling infection and outbreak, good commercialization development prospects, and good safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] In one embodiment of the present invention, the method for preparing an inactivated vaccine for preventing or treating duck spleen necrosis is provided, including the following steps:
[0043] (1) Duck reovirus with the deposit number CCTCC NO: V201843 was inoculated into the chicken liver cancer cell (LMH) cell line. After the duck reovirus was proliferated and cultured, the cells were broken by freezing and thawing, and the supernatant was collected , And purify to obtain the virus liquid of duck reovirus strain;
[0044] (2) Inactivate the virus liquid of the duck reovirus strain, add Tween-80 and mix it as the water phase, and mix the white oil, aluminum stearate and Span-80 as the oil phase. The water phase and the oil phase are in a volume ratio 1:2 is evenly mixed and emulsified to obtain an inactivated vaccine to prevent or treat duck spleen necrosis.
[0045] In the preparation method of the above-mentioned inactivated vaccine, the LMH cell line is used to culture th...
Embodiment 1
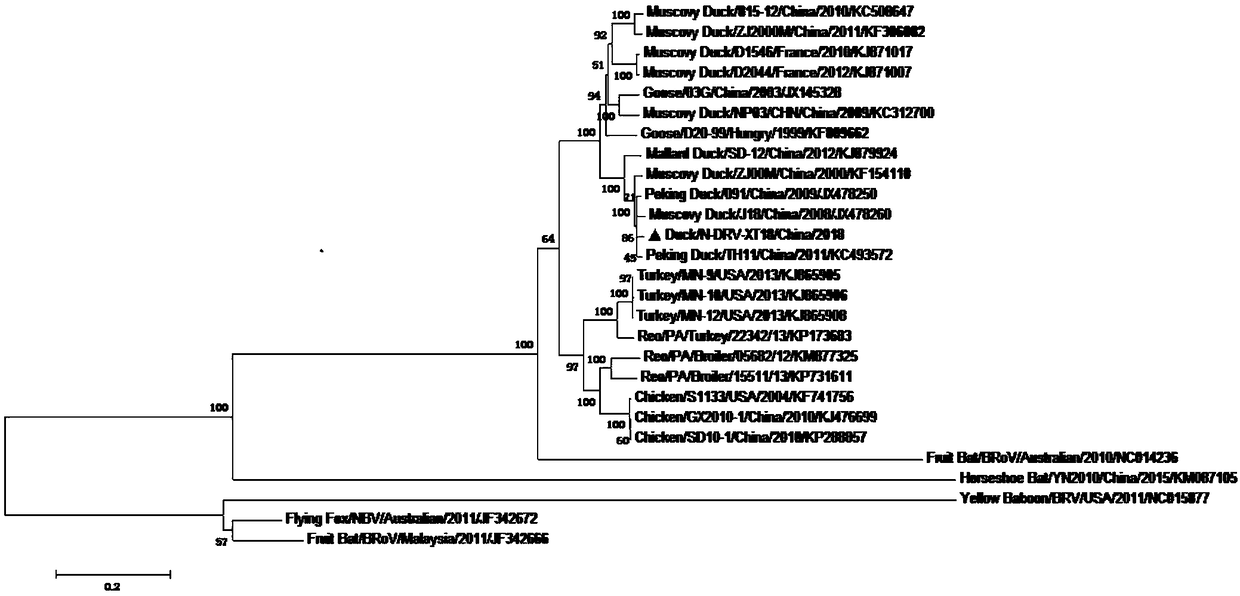
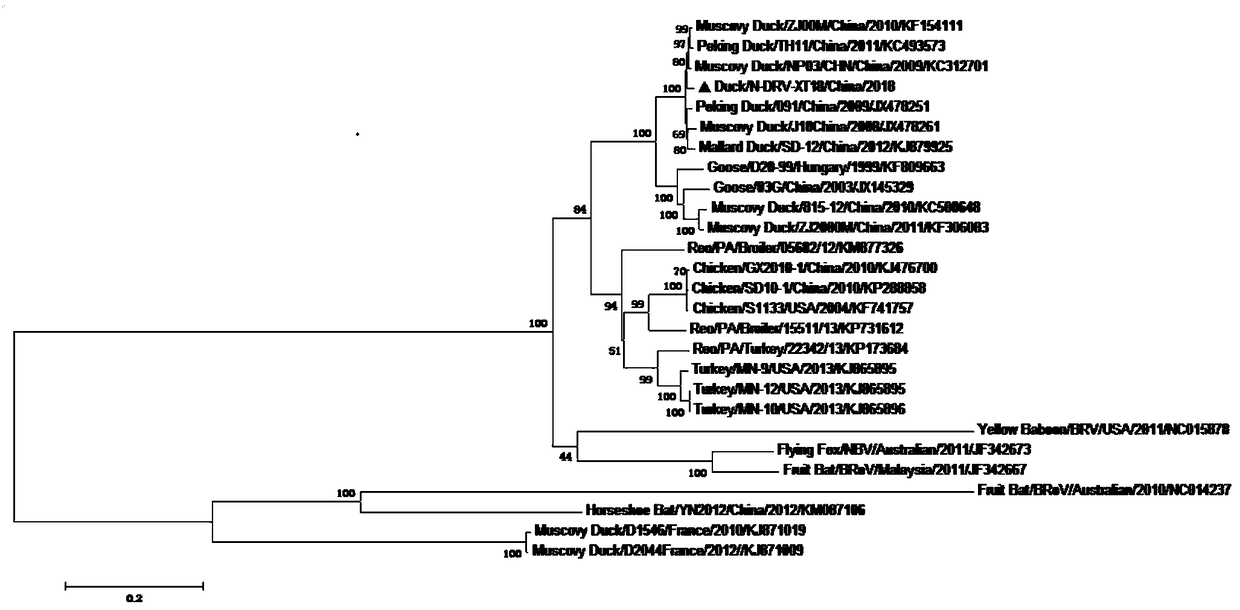
[0049] Example 1: Isolation and identification of duck reovirus strain
[0050] 1. Virus isolation:
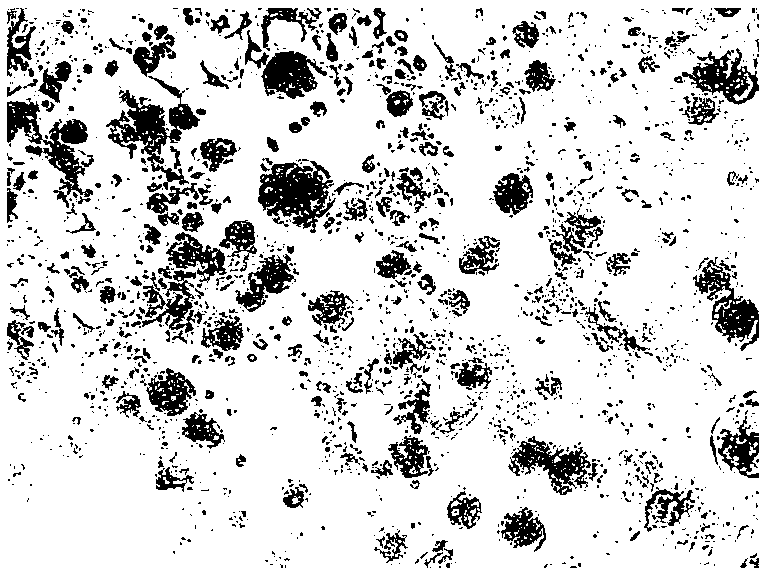
[0051] (1) Necrosis of the spleen tissue of diseased ducks in a duck farm in Tai'an City, Shandong Province was placed in a 15 mL centrifuge tube, and 5 times the volume of serum-free DMEM medium was added. After homogenization, it was repeatedly frozen and thawed 3 times, each time thawing After shaking on a shaker for 1-2 minutes, perform the next freeze-thaw; after freeze-thaw, centrifuge the 15mL centrifuge tube containing the sample in a centrifuge at 4000 rpm for 5 minutes; take the supernatant, filter it with a 0.22μm microporous membrane, and set aside ;
[0052] (2) According to the proliferation characteristics of reo, chicken liver cancer cells (LMH) were selected for virus isolation. According to the conventional cell culture method, wait for 25cm 2 When the cells in the cell flask cover the monolayer, aspirate the culture medium in the flask and wash the cells twice wi...
Embodiment 2
[0064] Example 2: Animal regression test
[0065] Take 40 healthy 1-day-old ducklings that have passed pathogen and antibody testing and do not carry new reovirus and virus antibodies. They are randomly divided into 2 groups with 20 in each group. Among them, the first group is the experimental group, the experimental group uses the fifth generation cell culture of the strain N-DRV-XT18 to be inoculated through the paw pad, 0.5mL / grass, the second group is the control group, each paw pad is inoculated with DMEM medium as Control, reared in isolation. After inoculation, the mental state of each group of animals and the spleen lesions were observed and recorded daily.
[0066] The experimental group began to develop splenic necrosis lesions 4 days after inoculation. On the 7th day after inoculation, 20 experimental groups all developed splenic necrosis lesions, which had the same symptoms as natural infection cases. The control group was in good health. The diseased spleen of the e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com