A catalyst-free hydrazone formation chemical modification method for polypeptides or proteins based on electron-deficient benzaldehydes
A technology for protein modification and connecting polypeptides, which is applied in the field of chemical modification of polypeptides and/or proteins, and can solve the problems of limited application range and limited number of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
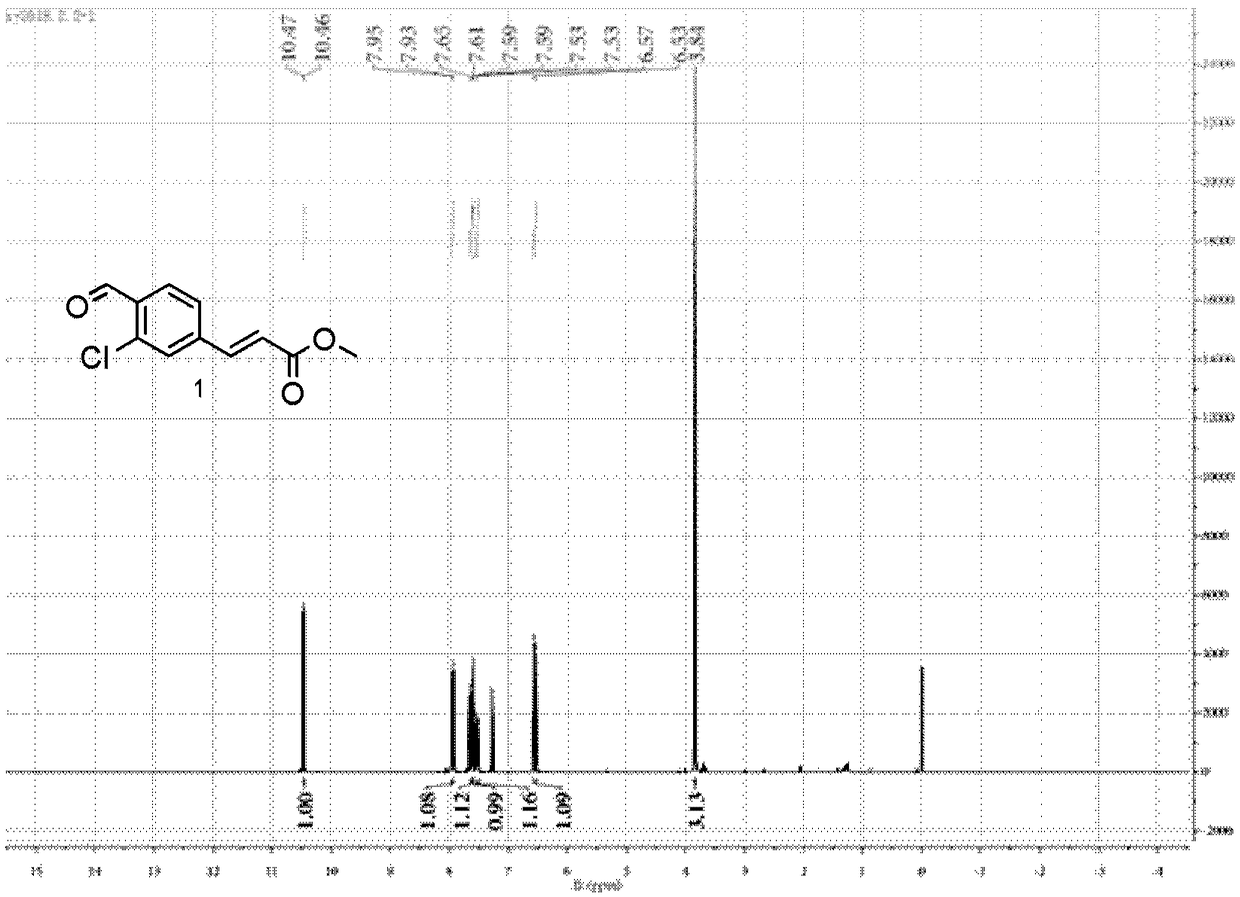
[0103] The electrophile shown in embodiment 1 preparation formula 1, 2 and 3
[0104] (1) The electrophile of formula 1 is prepared by chemical synthesis. The specific process is as follows:
[0105]
[0106] Commercially available 4-amino-2-chlorobenzonitrile (1.5 g, 10 mmol, purchased from TCI (Shanghai) Chemical Industry Development Co., Ltd.) was weighed and added to a 25 mL round bottom flask. Add 5mL of deionized water and 12mmol of concentrated hydrochloric acid to it. It should be noted that the concentrated hydrochloric acid needs to be added dropwise slowly. After the dropwise addition was complete, stirring was continued for 30 minutes. Sodium nitrite (0.76 g, 11 mmol) was then added to the flask and the reaction was stirred until the precipitate mostly disappeared (10 minutes or so). After observing this phenomenon, potassium iodide (1.82 g, 11 mmol) was added to the flask, and the stirring reaction was continued for 1 hour. After the reaction was completed,...
Embodiment 2
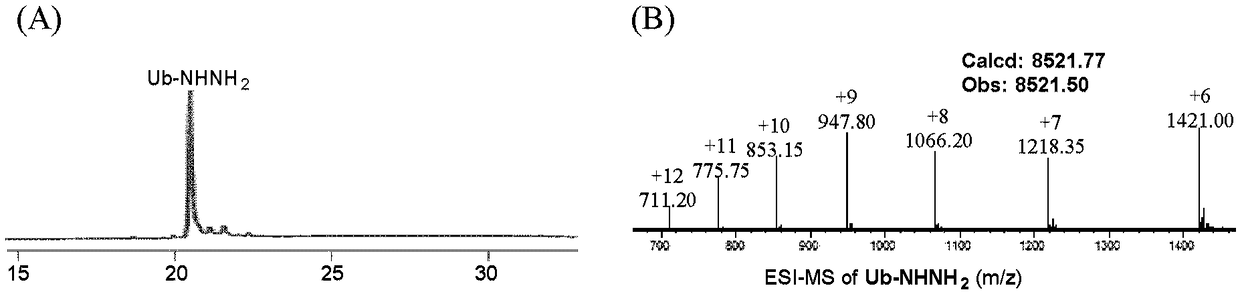
[0118] Embodiment 2 prepares the polypeptide hydrazone connection product of formula 9
[0119] The reaction formula of this embodiment is as follows:
[0120]
[0121] Concrete reaction steps are as follows:
[0122] (1) Dissolve the powder of polypeptide hydrazide (Leu-Tyr-Arg-Ala-Phe-NHNH2) in PME buffer (100mM PIPES, 1mM MgSO 4 , 2mM EGTA, pH 7.0) and prepared as a 1 mg / mL stock solution, the electron-deficient benzaldehyde compound 1 was dissolved in ethanol and prepared as a 10 mM stock solution.
[0123] (2) Take a 4mL plastic round bottom centrifuge tube and add 1mL of PME buffer into the tube. Then take an appropriate amount of the polypeptide hydrazide stock solution and the electron-deficient benzaldehyde 1 stock solution prepared in step (1) into the above-mentioned centrifuge tube, and the final concentrations of the two are 30 μM and 200 μM, respectively. Add a magnetic stir bar to the centrifuge tube and stir the reaction at room temperature for 30 minutes...
Embodiment 3
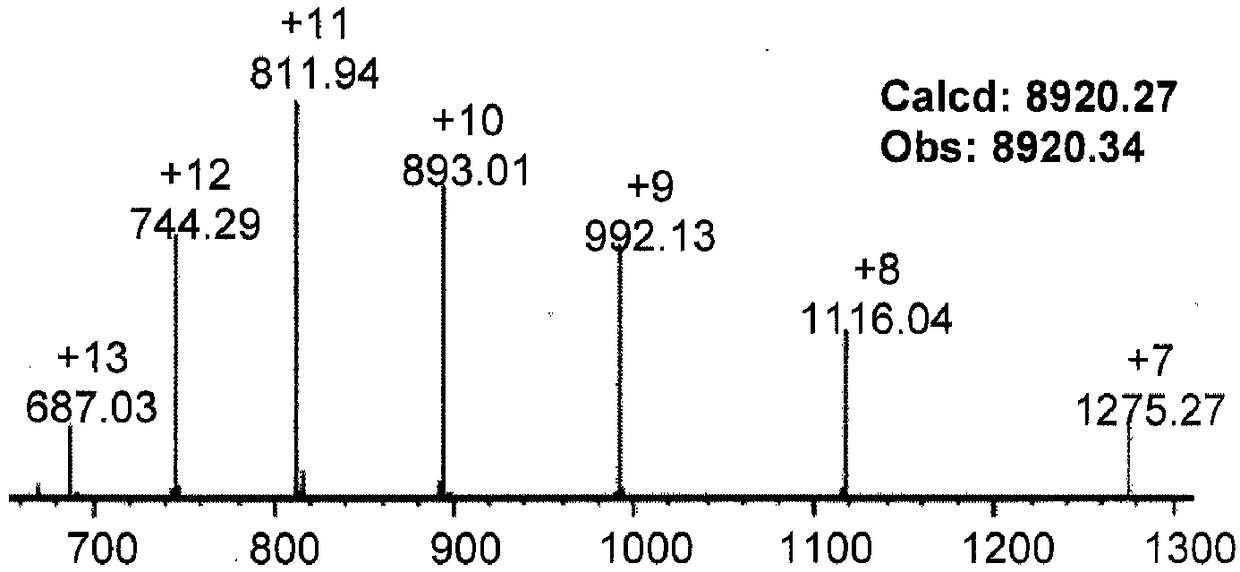
[0132] Embodiment 3 prepares the protein hydrazone connection product of formula 12
[0133] The reaction formula of this embodiment is as follows:
[0134]
[0135] This example can prove that the present invention is suitable for coupling between proteins and drug molecules, and may be applied in the field of antibody-drug coupling in the future.
[0136] Concrete reaction steps are as follows:
[0137] (1) Dissolve the powder of ubiquitin hydrazide in PME buffer (100mM PIPES, 1mMMgSO 4 , 2mM EGTA, pH 7.0) and prepared as a stock solution of 1mg / mL, the electron-deficient benzaldehyde (formula 3) coupled with dasatinib molecules was dissolved in N,N-dimethylformamide and prepared as 10 mg / mL stock solution.
[0138] (2) Take a 4mL plastic round bottom centrifuge tube and add 1mL of PME buffer into the tube. Then take an appropriate amount of the protein hydrazide stock solution prepared in step (1) and the electron-deficient benzaldehyde stock solution coupled with da...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com