Tripeptides with ace inhibitory activity and applications thereof
A technology for inhibiting activity and drugs, applied in tripeptides with ACE inhibitory activity and its application fields, can solve problems such as difficult and complex biologically active peptides, and achieve easy absorption, overcome digestion in the gastrointestinal tract, good potential and application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Virtual screening and activity verification of large yellow croaker-conactin ACE inhibitory peptide
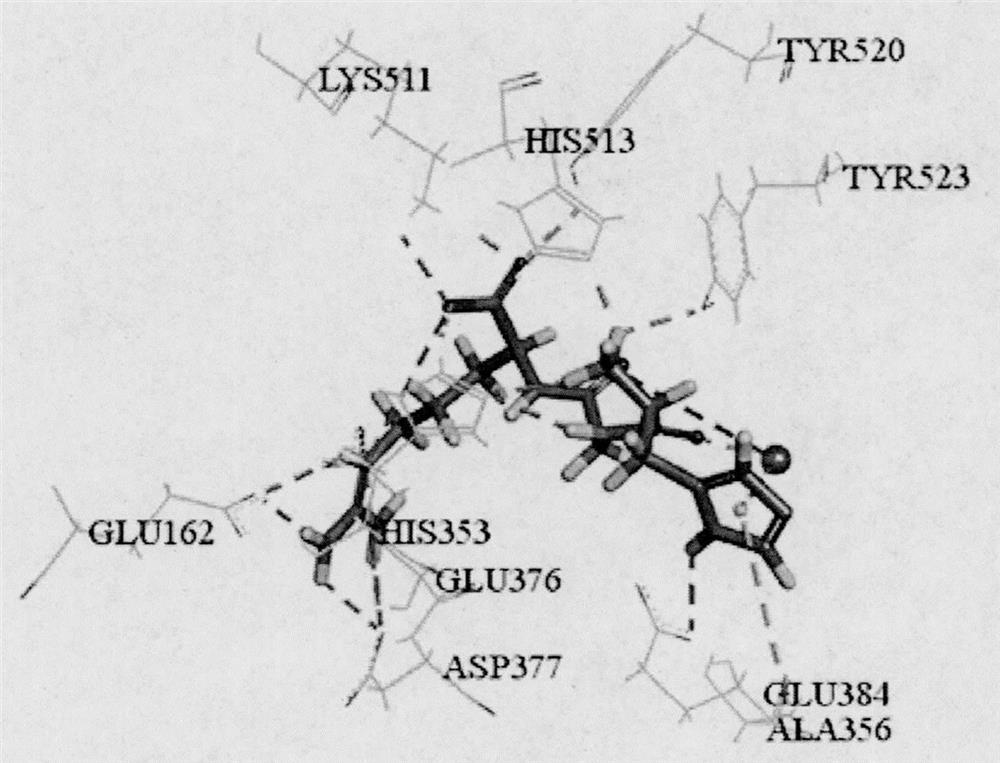
[0027] Based on the online program ExPASy PeptideCutter, three typical gastrointestinal digestive proteases, pepsin (EC 3.4.23.1), trypsin (EC 3.4.21.4) and chymotrypsin (EC 3.4.21.1) were used to treat large yellow croaker muscle Kinetin (Accession of NCBI: KKF11904.1) was subjected to simulated enzymatic hydrolysis. ToxinPred, peptide property calculator, PeptideRanker and admetSAR were used to predict toxicity, water solubility, biological activity and ADME properties of unreported tripeptide sequences. A tripeptide with non-toxicity, good solubility, activity score higher than 0.5 and good intestinal permeability was screened, and further screened by molecular docking with ACE (PDB ID: 1O86) as the protein target. Combined with the key amino acids of CDOCKER score and function, the tripeptide HGR with theoretical potential ACE inhibitory activity was scre...
Embodiment 2
[0028] Example 2 Virtual screening and activity verification of ACE inhibitory peptide of large yellow croaker moesin
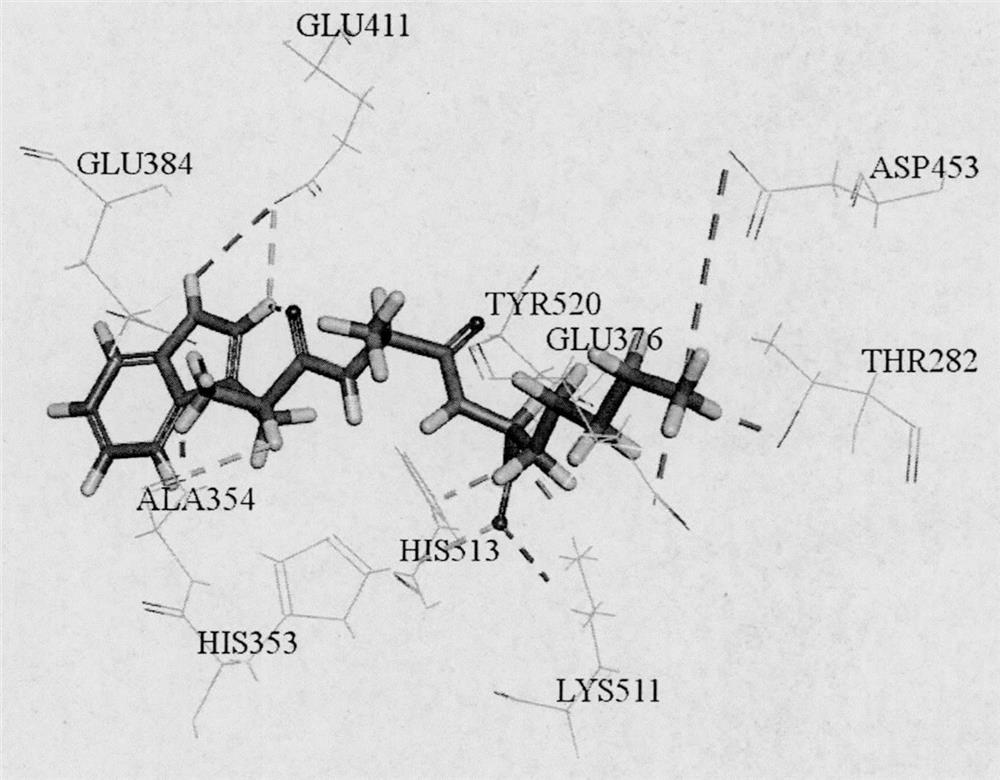
[0029] Based on ExPASy PeptideCutter, an online virtual enzyme digestion tool, pepsin (EC3.4.23.1) and trypsin (EC 3.4.21.4) were used to perform virtual enzyme digestion on the moesin of large yellow croaker (Accession of NCBI: KKF16686), and passed The online tools ToxinPred, peptide property calculator, PeptideRanker and admetSAR predict the toxicity, water solubility, biological activity and ADME properties of unreported tripeptide sequences. A tripeptide with non-toxicity, good solubility, activity score higher than 0.5 and good intestinal permeability was screened, and further screened by molecular docking with ACE (PDB ID: 1O86) as the protein target. Combined with the key amino acids of CDOCKER score and function, the tripeptides WAK and CMK with theoretical potential ACE inhibitory activity were screened. The results showed that WAK could bind to th...
Embodiment 3
[0030] Example 3 Virtual screening and activity verification of large yellow croaker collagen ACE inhibitory peptide
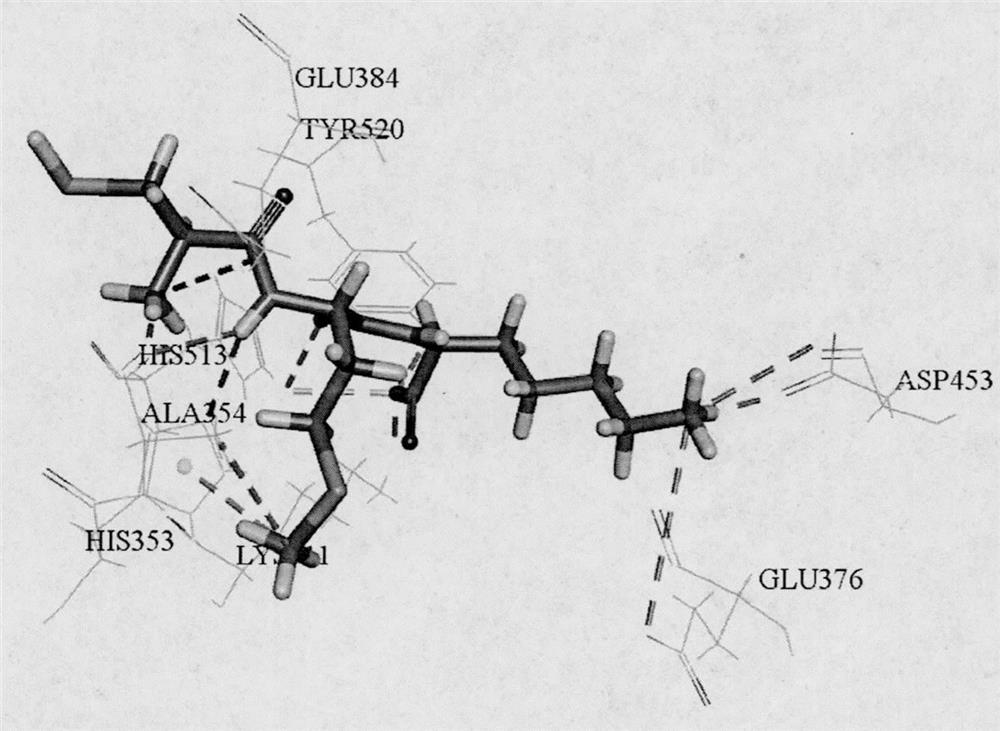
[0031] Based on the ExPASy PeptideCutter online virtual digestion tool, three typical gastrointestinal digestive proteases, pepsin (EC 3.4.23.1), trypsin (EC 3.4.21.4) and chymotrypsin (EC 3.4.21.1) were used to Yellow croaker collagen (Accession of NCBI: KKF14511) was subjected to virtual digestion, and the toxicity, water solubility, biological activity and ADME properties of unreported tripeptide sequences were predicted by online tools ToxinPred, peptide property calculator, PeptideRanker and admetSAR. A tripeptide with non-toxicity, good solubility, activity score higher than 0.5 and good intestinal permeability was screened, and further screened by molecular docking with ACE (PDB ID: 1O86) as the protein target. Combined with the key amino acids of CDOCKER score and function, the tripeptides AMR and LEW with theoretical potential ACE inhibitory activity ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com