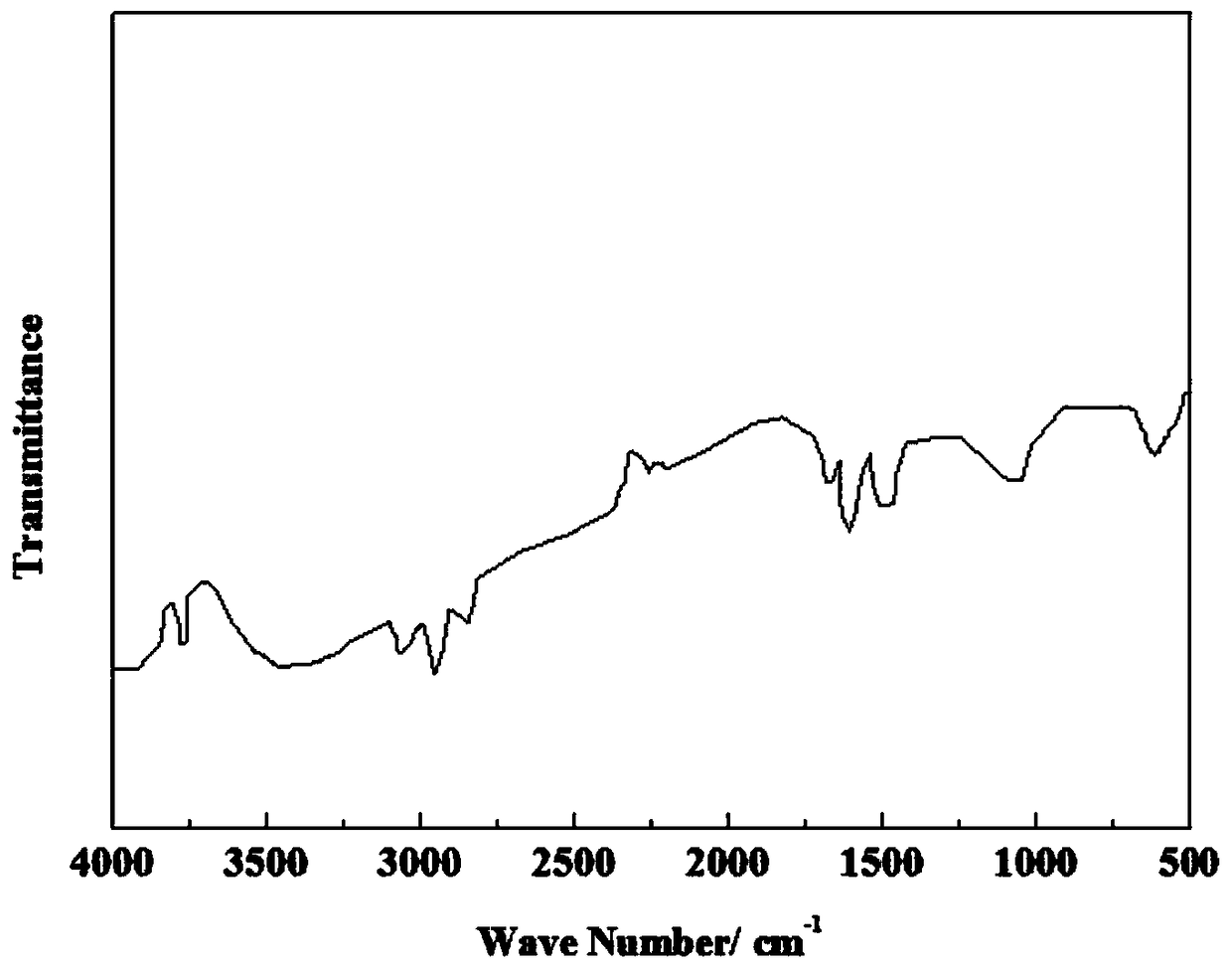
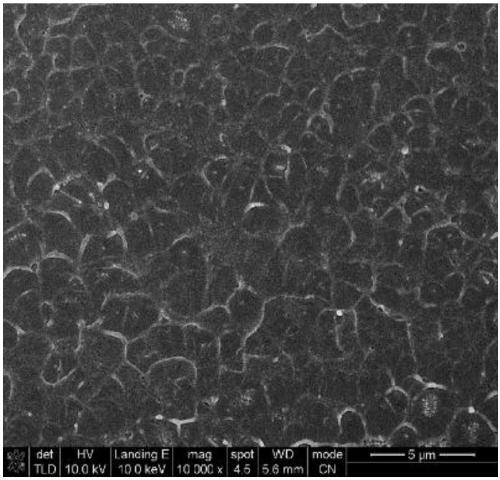
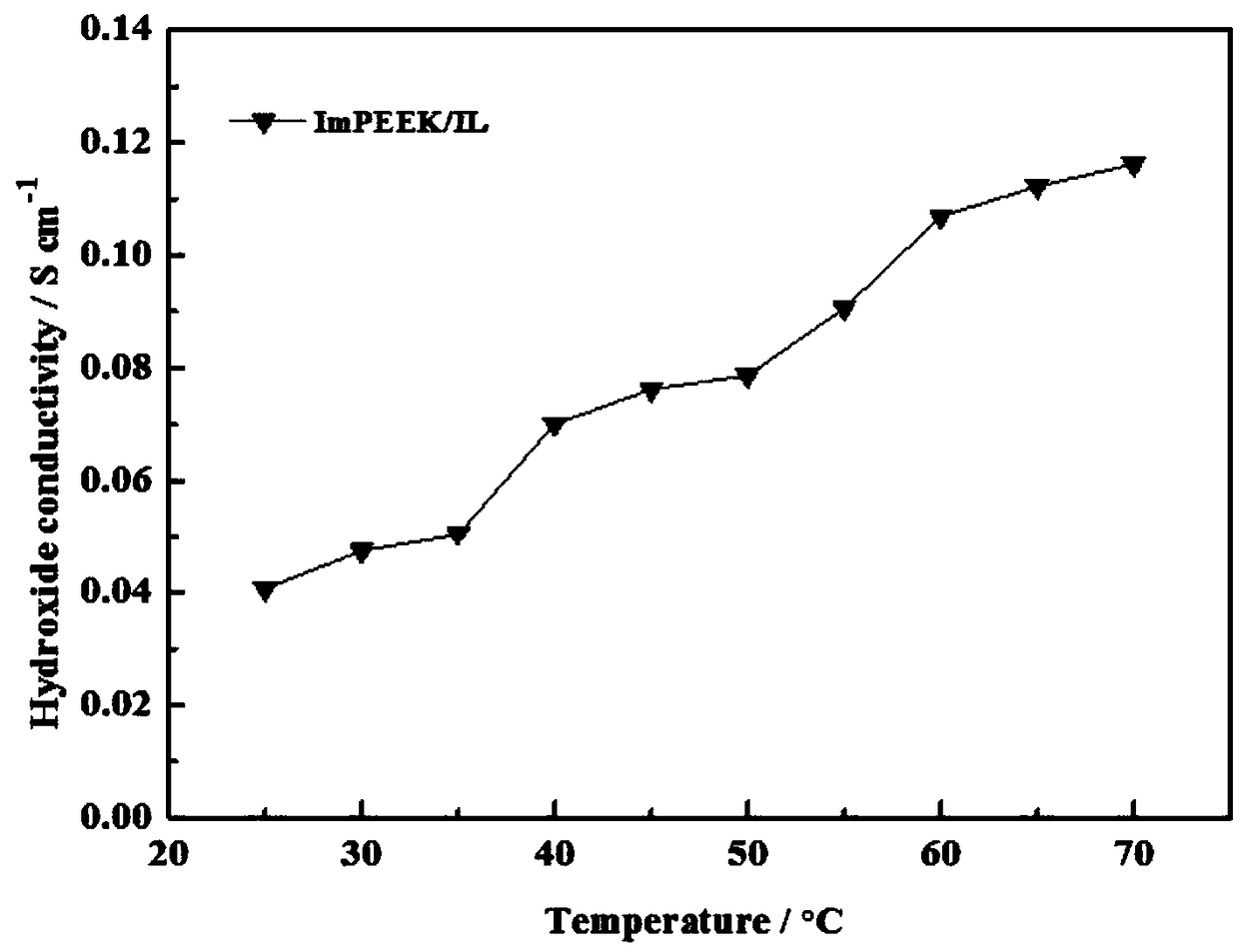
Imidazole ionic liquid and preparation method and application thereof
An ionic liquid and imidazole technology is applied in the preparation of anion exchange membranes. The preparation of imidazole ionic liquids can solve the problems of poor stability of anion exchange membranes, poor membrane performance improvement effect, and low ionic conductivity. Achieve the effect of improving mechanical stability, improving ion conductivity and good ion conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] in N 2 Under the protection of 250ml three-neck round bottom flask, add 0.25mol potassium carbonate, 0.5mol 2-methylimidazole and 0.6mol acrylonitrile to 60mL anhydrous methanol, reflux and stir in 60°C oil bath for 12h, the reaction is complete ; After the reaction was stopped, unreacted raw materials and methanol were removed by rotary evaporation, and dried at 70°C for 24 hours under vacuum to obtain a yellow transparent liquid, which was 1-nitrile ethyl-2-methylimidazole. The yield was 82%.
[0048] in N 2 Under protection, in a 250ml three-necked round-bottomed flask, the synthesized 1-nitrile ethyl-2-methylimidazole (about 0.4mol) was mixed with 3-bromopropylamine hydrobromide in a molar ratio of 1:1, and dissolved In 50mL of anhydrous methanol, stir at reflux at 80°C for 24h. Methanol was distilled off under reduced pressure at 50°C. The obtained 0.34mol ionic liquid was diluted with 3ml of deionized water, and then 10% KOH solution was added to adjust the pH...
Embodiment 2
[0057] in N 2 Under the protection of 250ml three-neck round bottom flask, add 0.25mol potassium carbonate, 0.5mol 2-methylimidazole and 0.5mol chloroacetonitrile to 60mL anhydrous methanol, reflux and stir in 55℃ oil bath for 10h, the reaction is complete ; After the reaction was stopped, unreacted raw materials and solvents were removed by rotary evaporation, and dried at 60° C. under vacuum for 24 hours to obtain a light yellow transparent liquid, which was 1-nitrile methyl-2-methylimidazole. The yield was 80%.
[0058] in N 2 Under protection, in a 250ml three-neck round bottom flask, the synthesized 1-nitrile methyl-2-methylimidazole (about 0.4mol) was mixed with 3-bromopropylamine hydrobromide in a molar ratio of 1:1, and dissolved In 50mL of anhydrous methanol, stir at reflux at 80°C for 24h. Methanol was distilled off under reduced pressure at 50°C. The obtained 0.34mol ionic liquid was diluted with 3ml of deionized water, and then 10% KOH solution was added to adj...
Embodiment 3
[0062] in N 2 Under the protection of 250ml three-neck round bottom flask, add 0.25mol potassium carbonate, 0.5mol 2-methylimidazole and 0.6mol acrylonitrile to 60mL anhydrous methanol, reflux and stir in 60°C oil bath for 12h, the reaction is complete ; After stopping the reaction, unreacted raw materials and solvents were removed by rotary evaporation, and dried at 70° C. under vacuum for 24 hours to obtain a yellow transparent liquid, which was 1-nitrile ethyl-2-methylimidazole. The yield was 82%.
[0063] in N 2 Under protection, in a 250ml three-necked round-bottomed flask, the synthesized 1-nitrile ethyl-2-methylimidazole (about 0.4mol) was mixed with 2-bromoethylamine hydrobromide in a molar ratio of 1:1.1, Dissolve in 50mL of anhydrous methanol, and stir at reflux at 75°C for 24h. Methanol was distilled off under reduced pressure at 50°C. The obtained 0.33mol ionic liquid was diluted by adding 3ml of deionized water, then adding KOH solution with a mass fraction of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com