Separation method and separation matrix
A technology for separating matrices and matrices, which is used in the ion exchange separation of proteins, separation matrices with high alkali stability, and the preparation of such matrices, which can solve problems such as changing pH.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0080] The grafting of embodiment 1.1-vinyl-2-pyrrolidone (VP) and vinylsulfonic acid (VSA)
[0081] 1a) Allylation of Sepharose Bead Support
[0082] 50 g of highly rigid, drained agarose beads (prepared according to the method described in US6602990) were added to a 500 ml round bottom flask and mixed by stirring in 101.4 g of 50% aqueous NaOH and 77.04 g of distilled water. The temperature was raised to 50°C for 30 minutes. Thereafter, allyl glycidyl ether (AGE) was added in an amount to produce the AGE / reactant volume ratio according to Table 2, and the reaction was continued overnight at 50° C. for 17 hours. The beads were then filtered off and washed with ethanol (5x300ml) followed by distilled water (5x300ml).
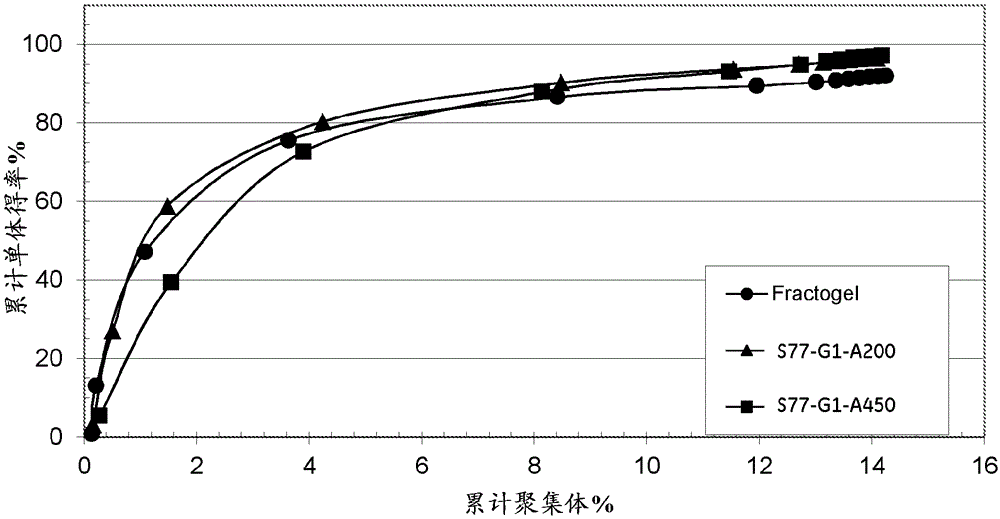
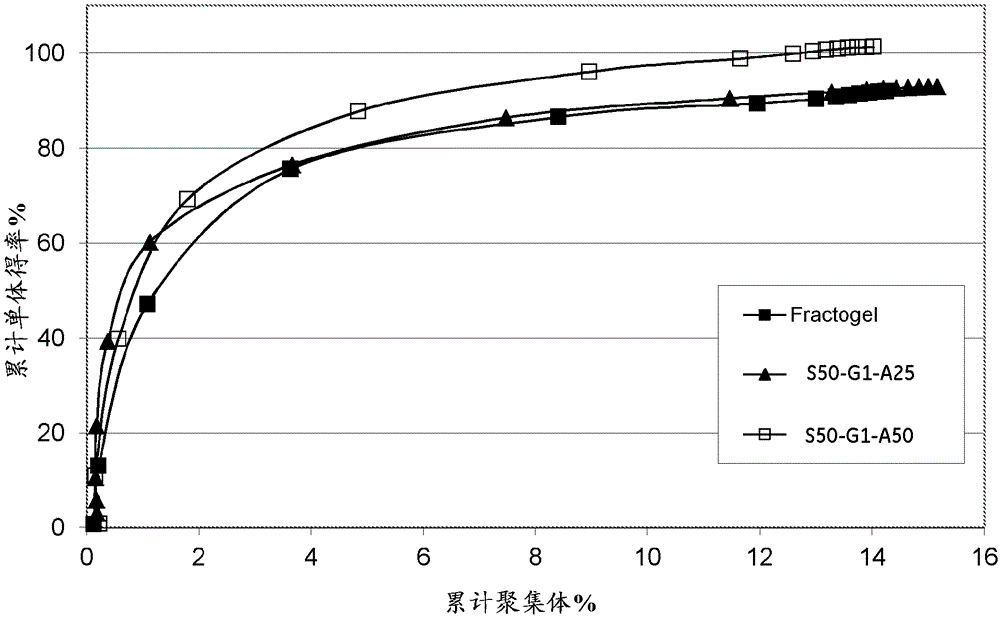
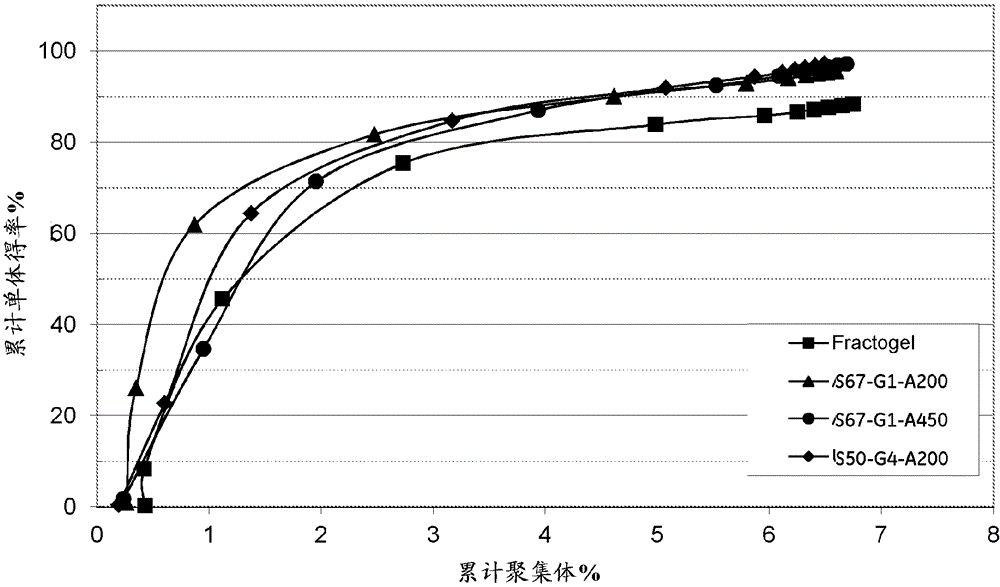
[0083] Agarose beads of 4 different pore sizes were used, corresponding to Kd values of 0.50, 0.67, 0.77 and 0.80 for dextran Mw110 kDa. Using an AKTA equipped with an A-900 autosampler TM The detector is evaluated for Kd. Connect Shimadzu RI-Detector (RI...
Embodiment 2
[0093] Example 2. Evaluation of prototypes grafted with VP and VSA
[0094] Column Packing and Testing
[0095] Prototype media was settled and compressed in Tricorn 5 / 50 columns. After filling, the column was tested in 0.4M NaCl at 0.065 ml / min by injecting 10 μl of 2M NaCl with 3% (v / v) acetone. Registration A 280 、A 260 and conductance peaks and integrated.
[0096] An acceptable criterion for a successful filling is an asymmetry value between 0.8 and 1.5.
[0097] Mab sample preparation
[0098] The protein A purified Mab was buffer-exchanged to 50 mM sodium acetate + 10 mM NaCl (pH 5.25) using a HiPrep Desalting 26 / 10 column. Protein concentrations were then determined spectrophotometrically.
[0099] by A 280 concentration determination
[0100] Protein concentration was determined spectrophotometrically at 280 nm using Lambert Beer's law (Equation 1).
[0101] Equation 1: C=A / (l*ε)
[0102] where C is the concentration (mg / ml), l is the path length ...
Embodiment 3
[0138] Example 3. Grafting of VP and 3-allyloxy, 2-hydroxyl-1-propanesulfonic acid (APS)
[0139] Place the agarose beads on a glass filter and wash several times with 40% APS solution (2x gel volume). The suspension was sucked dry, after which 75 g were added to a 250 ml three necked round bottom flask. 1.5 g ADBA was added to the flask along with 30 g APS solution and 25.5 ml VP. Polymerization was started at 65°C and stirred for about 17h. Wash the gel thoroughly with distilled water.
[0140] Measurement of Static Binding Capacity in 96-well Filter Plates
[0141] Using a pre-programmed Gilson automaton, 1% gel slurries were prepared and filled into 96-well filter plates corresponding to 2 microliters of settled gel / well.
[0142] Equilibration of the gel filled in the plate was performed by adding 200 microliters of equilibration buffer from the plate prepared in the Tecan automaton, followed by agitation at 1100 rpm for 1 minute, after which the buffer was removed by v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com