Polymers Containing Brominated Alkylfluorenes in Main Chain and Their Applications in Anion Exchange Membranes
A technology of bromoalkylfluorene and bromoalkyl, which is applied in the field of polymer material synthesis, can solve problems such as limiting the practical application of materials, and achieve the effects of good tolerance, simplicity, convenience and cost, and high alkali stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
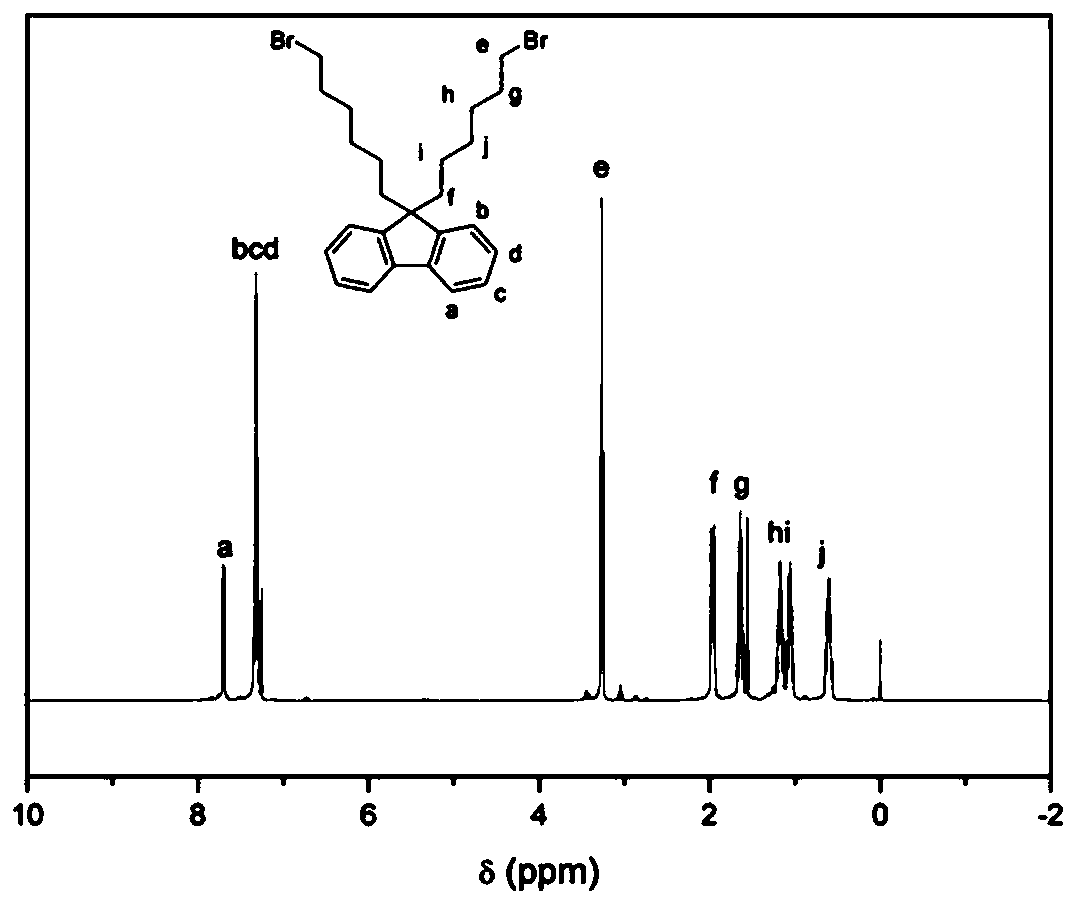
[0034] Synthesis of 9,9-dibromohexylfluorene
[0035] In this example, the dibromoalkylfluorene is illustrated by taking 9,9-dibromohexylfluorene as an example, and all other dibromoalkylfluorenes that meet the requirements of the structural formula of the present invention fall within the scope of the present invention.
[0036] According to the reported synthetic route, the synthesis of 9,9-dibromohexylfluorene was achieved by alkylation of fluorene by direct C–H alkylation under aqueous conditions. Fluorene (3.3 g, 20 mmol), 15 mL of 50% aqueous NaOH, 1,6-dibromohexane (34 g, 140 mmol) and a catalytic amount of tetrabutylammonium iodide (0.74 g, 10 mol%) were added to the flask. The flask was degassed three times by applying refrigeration cycle pumping. The reaction mixture was continuously heated at 70°C for 4 hours, cooled to room temperature and extracted with chloroform. The organic layer was washed with water and dried over anhydrous sodium sulfate. The solvent was ...
Embodiment 2
[0038] Synthesis of Homopolymer (PF) Containing Brominated Alkyl Fluorene in Main Chain
[0039] Dissolve dibromoalkylfluorene and trifluoroacetone (molar ratio 1:1.1) in dichloromethane, cool in an ice bath to zero, add trifluoromethanesulfonic acid, react at low temperature for 30 minutes, remove the ice bath, and react at room temperature for two After 1 hour it was diluted with dichloromethane and precipitated in methanol. Suction filtration, washing with methanol to obtain the final product. (yield 97%)
Embodiment 3
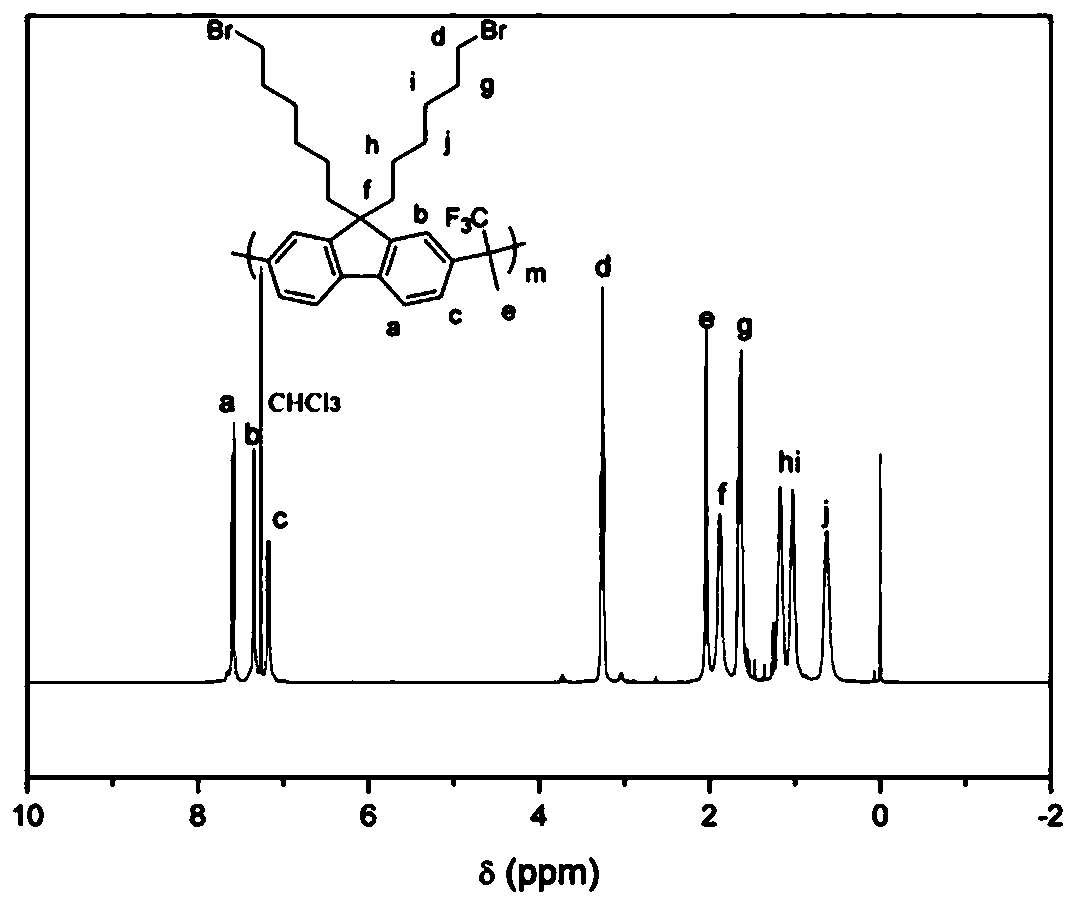
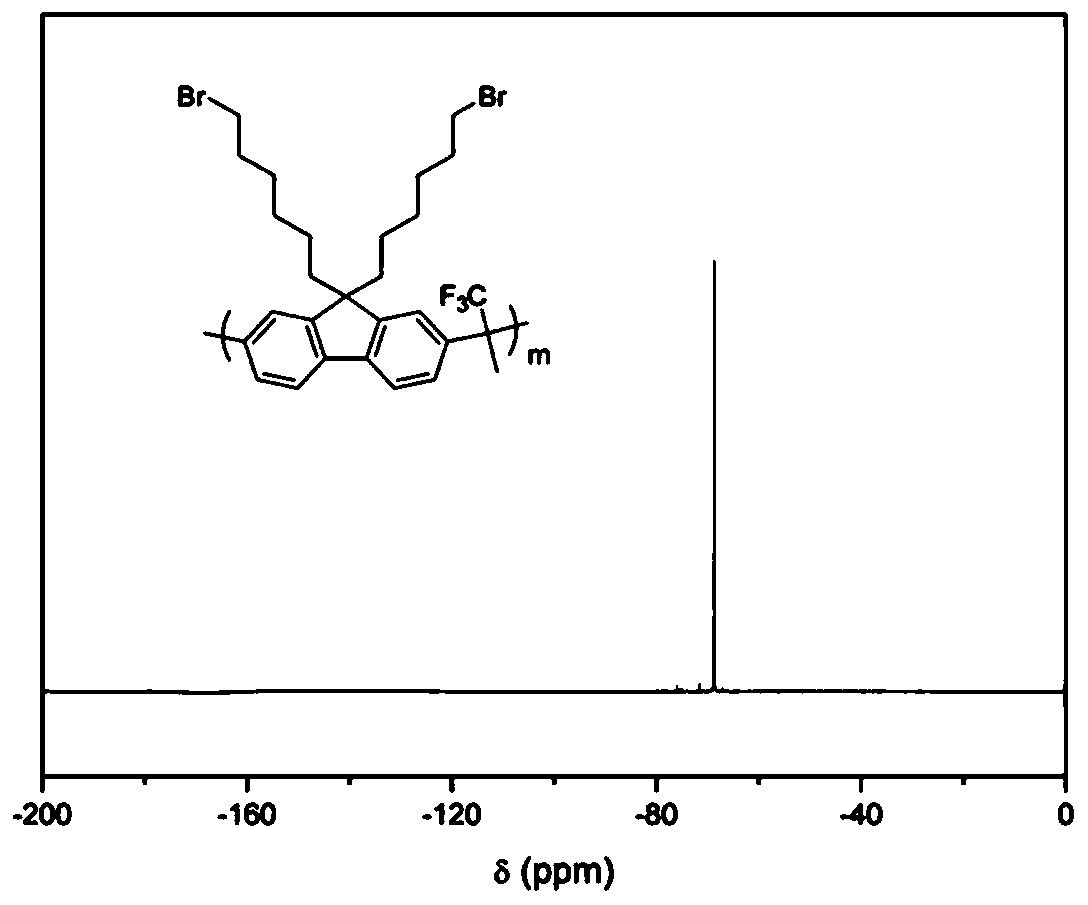
[0041] Synthesis of Main Chain Containing Brominated Alkyl Fluorene Copolymer (PBF)
[0042] Dissolve dibromoalkylfluorene, biphenyl and trifluoroacetone (molar ratio 1:1:2.2) in dichloromethane, cool to zero in an ice bath, add trifluoromethanesulfonic acid, react at low temperature for 30 minutes, remove the ice bath, reacted at room temperature for two hours, diluted with dichloromethane, and precipitated in methanol. Suction filtration, washing with methanol to obtain the final product. (yield 95%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com