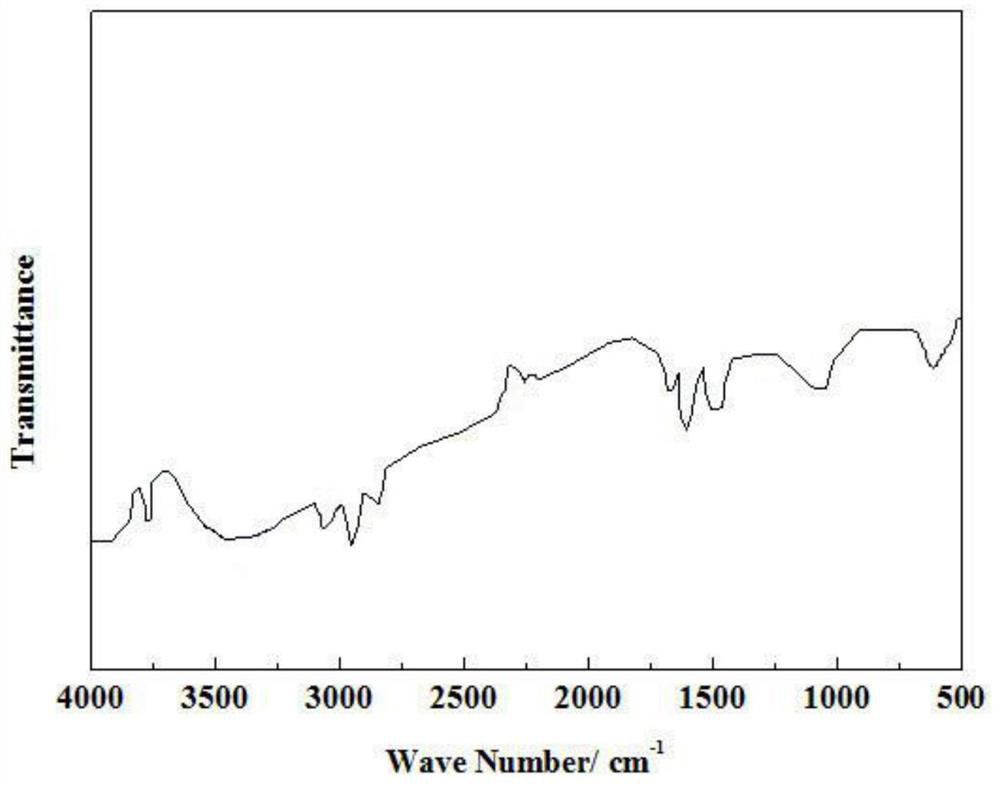
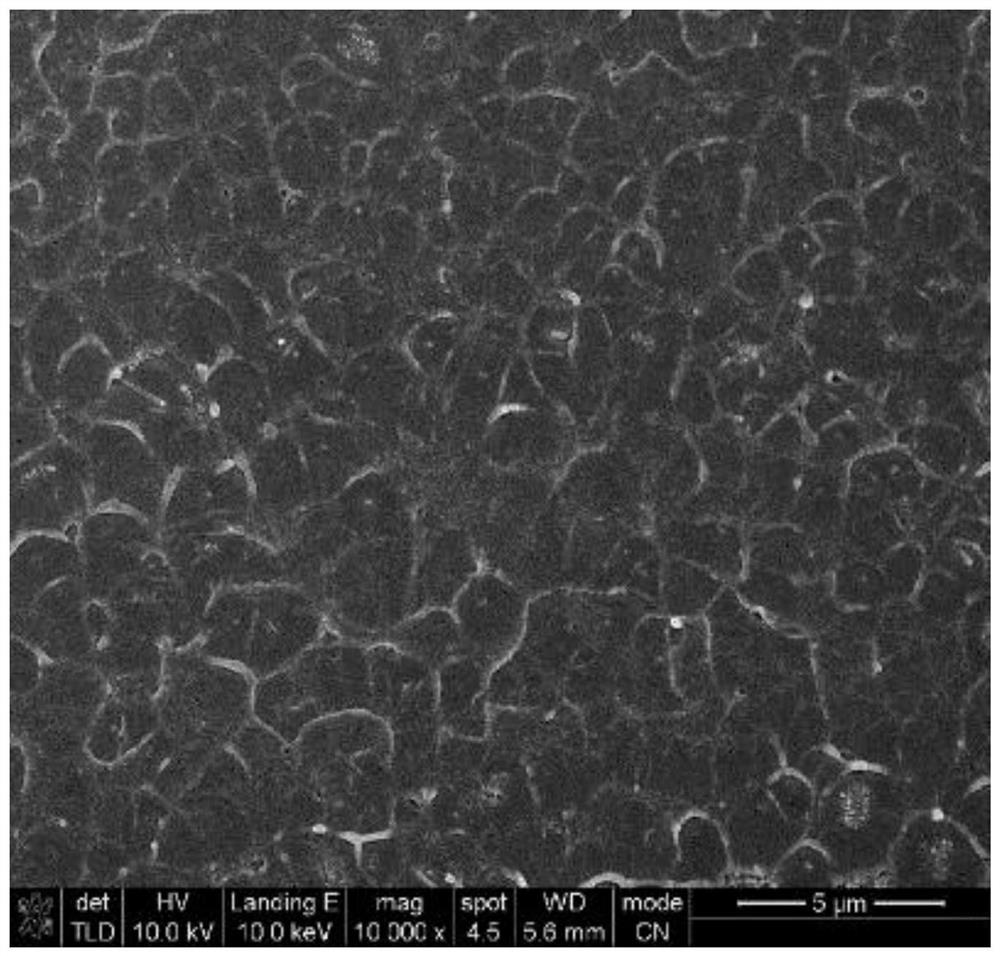
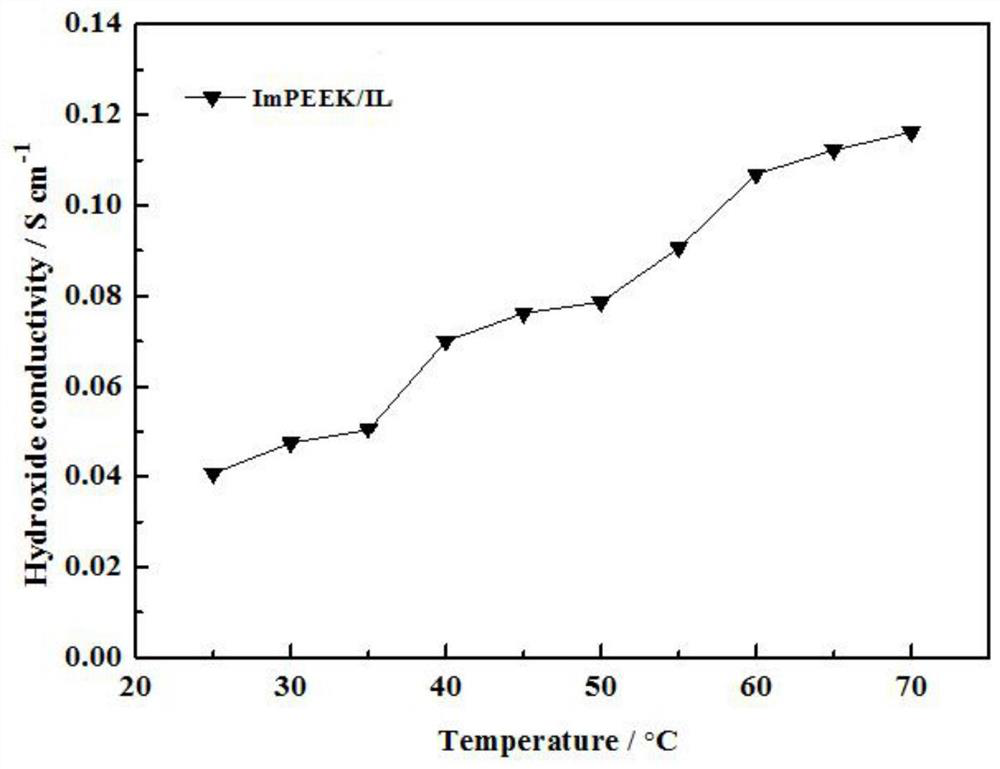
A kind of imidazole ionic liquid and its preparation method and application
A technology of ionic liquids and imidazoles, applied in the preparation of anion-exchange membranes, in the field of preparation of imidazole-based ionic liquids, can solve the problems of poor stability of anion-exchange membranes, poor membrane performance improvement, and low ion conductivity. Achieve the effect of improving mechanical stability, improving ion conductivity, and good ion conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] in N 2 Under the protection of 250ml three-neck round bottom flask, add 0.25mol potassium carbonate, 0.5mol 2-methylimidazole and 0.6mol acrylonitrile to 60mL anhydrous methanol, reflux and stir in 60°C oil bath for 12h, the reaction is complete ; After the reaction was stopped, unreacted raw materials and methanol were removed by rotary evaporation, and dried at 70°C for 24 hours under vacuum to obtain a yellow transparent liquid, which was 1-nitrile ethyl-2-methylimidazole. The yield was 82%.
[0049] in N 2 Under protection, in a 250ml three-necked round-bottomed flask, the synthesized 1-nitrile ethyl-2-methylimidazole (about 0.4mol) was mixed with 3-bromopropylamine hydrobromide in a molar ratio of 1:1, and dissolved In 50mL of anhydrous methanol, stir at reflux at 80°C for 24h. Methanol was distilled off under reduced pressure at 50°C. The obtained 0.34mol ionic liquid was diluted with 3ml of deionized water, and then 10% KOH solution was added to adjust the pH...
Embodiment 2
[0058] in N 2 Under the protection of 250ml three-neck round bottom flask, add 0.25mol potassium carbonate, 0.5mol 2-methylimidazole and 0.5mol chloroacetonitrile to 60mL anhydrous methanol, reflux and stir in 55℃ oil bath for 10h, the reaction is complete ; After the reaction was stopped, unreacted raw materials and solvents were removed by rotary evaporation, and dried at 60° C. under vacuum for 24 hours to obtain a light yellow transparent liquid, which was 1-nitrile methyl-2-methylimidazole. The yield is 80%.
[0059] in N 2 Under protection, in a 250ml three-neck round bottom flask, the synthesized 1-nitrile methyl-2-methylimidazole (about 0.4mol) was mixed with 3-bromopropylamine hydrobromide in a molar ratio of 1:1, and dissolved In 50mL of anhydrous methanol, stir at reflux at 80°C for 24h. Methanol was distilled off under reduced pressure at 50°C. The obtained 0.34mol ionic liquid was diluted with 3ml of deionized water, and then 10% KOH solution was added to adju...
Embodiment 3
[0063] in N 2 Under the protection of 250ml three-neck round bottom flask, add 0.25mol potassium carbonate, 0.5mol 2-methylimidazole and 0.6mol acrylonitrile to 60mL anhydrous methanol, reflux and stir in 60°C oil bath for 12h, the reaction is complete ; After stopping the reaction, unreacted raw materials and solvents were removed by rotary evaporation, and dried at 70° C. under vacuum for 24 hours to obtain a yellow transparent liquid, which was 1-nitrile ethyl-2-methylimidazole. The yield was 82%.
[0064] in N 2 Under protection, in a 250ml three-necked round-bottomed flask, the synthesized 1-nitrile ethyl-2-methylimidazole (about 0.4mol) was mixed with 2-bromoethylamine hydrobromide in a molar ratio of 1:1.1, Dissolve in 50mL of anhydrous methanol, and stir at reflux at 75°C for 24h. Methanol was distilled off under reduced pressure at 50°C. The obtained 0.33mol ionic liquid was diluted by adding 3ml of deionized water, then adding KOH solution with a mass fraction of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com