Wastewater defluorination application of iminodisuccinic acid (IDS) metal chelating type adsorbent
A technology of metal chelation and adsorbent, which is applied in the direction of adsorption of water/sewage treatment, water pollutants, water/sewage treatment, etc. It can solve the problems of poor stability, low removal rate, and small adsorption amount, and achieve a significant effect of fluoride removal , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
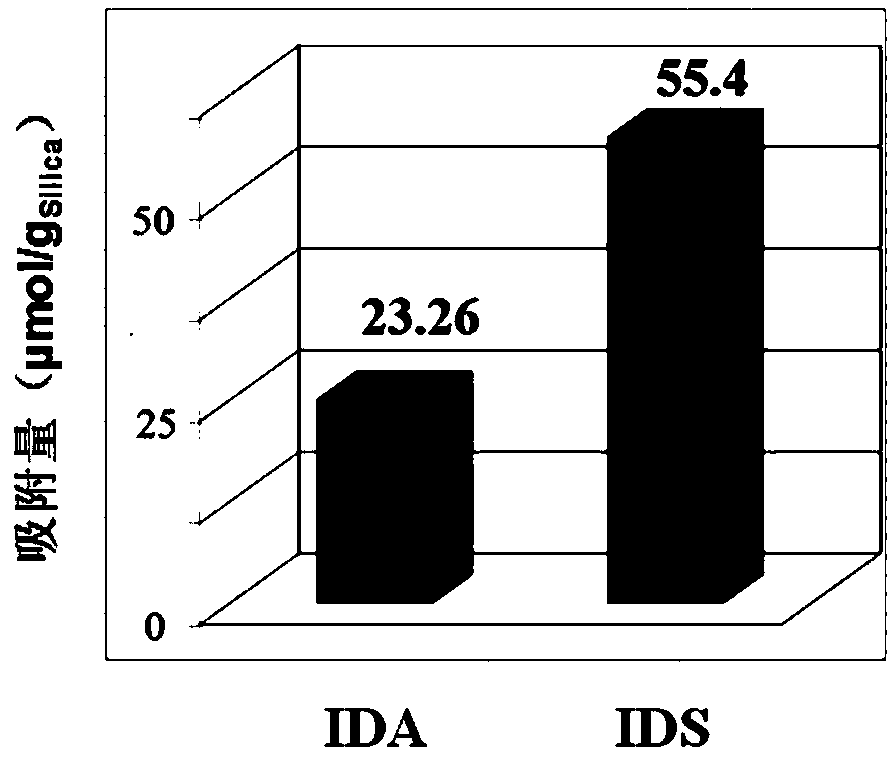
[0027] Example 1 Metal Fe 3+ Comparison experiment of adsorption capacity on IDS-silica gel chelating adsorbent and IDA-silica gel chelating adsorbent
[0028] IDS-Fe used in the present invention 3+ - Silica gel chelating adsorbent and IDA-Fe 3+ - Silica gel chelating adsorbent, IDA-Fe 3+ -The preparation process of styrene divinylbenzene chelated adsorbent is as follows:
[0029] Connect the IDS-silica gel column, IDA-silica gel column and IDA-styrene divinylbenzene column to the chromatographic system respectively. After washing with water, inject 0.05mol / L FeCl with a pump at a flow rate of 0.5mL / min 3 NaAc-HAc buffer solution (pH=4.0) until saturated. Then let it stand for 25min, and wash off the unbound metal ions with ultrapure water and 0.02mol / L phosphate buffer in sequence 2 S test), and then washed with ultrapure water to prepare IDS-Fe respectively 3+ - Silica metal chelate adsorbent, IDA-Fe 3+ - Silica metal chelate adsorbent and IDA-Fe 3+ - Styrene divin...
Embodiment 2
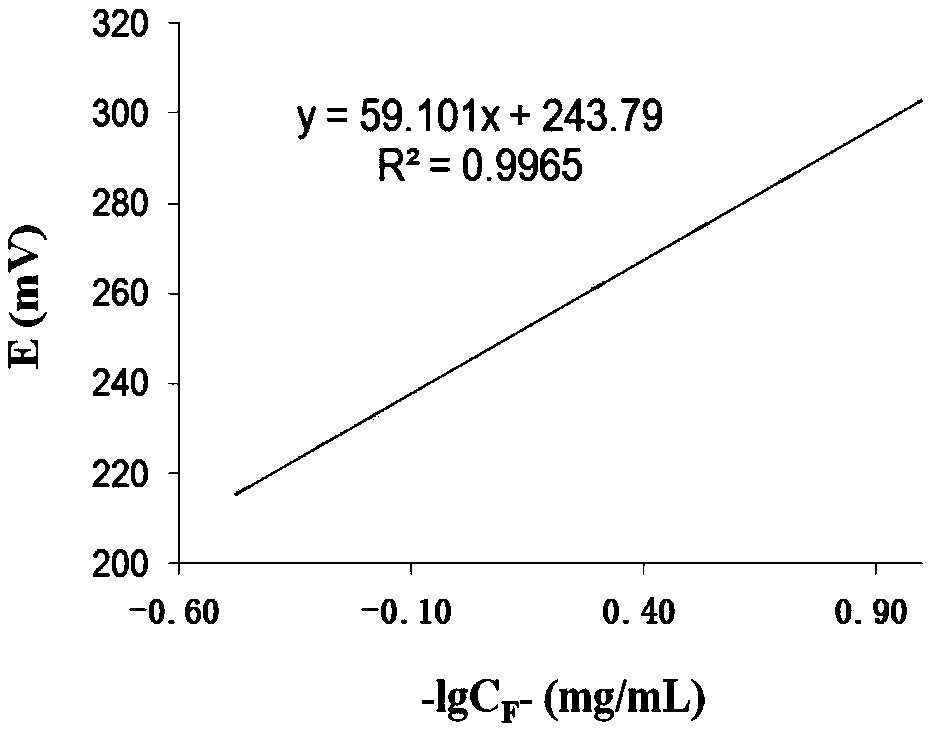
[0035] Embodiment 2 adopts the ion selective electrode method to draw F - Standard curve between content and potential value
[0036] (1) Preparation of fluoride standard solution [C F - =1mg / mL]: Sodium fluoride (NaF) was dried at 500°C for about 40 minutes, cooled naturally, weighed 0.2210g, dissolved completely with ultrapure water, diluted to 100mL, constant volume, and stored in a polyethylene bottle.
[0037] (2) Fluoride standard use solution [C F - =10 μg / mL]: Take 5.00 mL of the standard preparation solution in (1) with a pipette gun, dissolve it completely with ultrapure water, and set the volume to 500 mL.
[0038](3) Sodium hydroxide solution (400g / L): Weigh 40g of sodium hydroxide (NaOH), dissolve it completely with ultrapure water and set the volume to 100mL;
[0039] (4) Glacial acetic acid solution (C 冰醋酸 =1.06g / mL)
[0040] (5) Total ionic strength adjustment buffer solution (TISAB): weigh 59g sodium chloride (NaCl), weigh 3.48g trisodium citrate (NaCl ...
Embodiment 3I
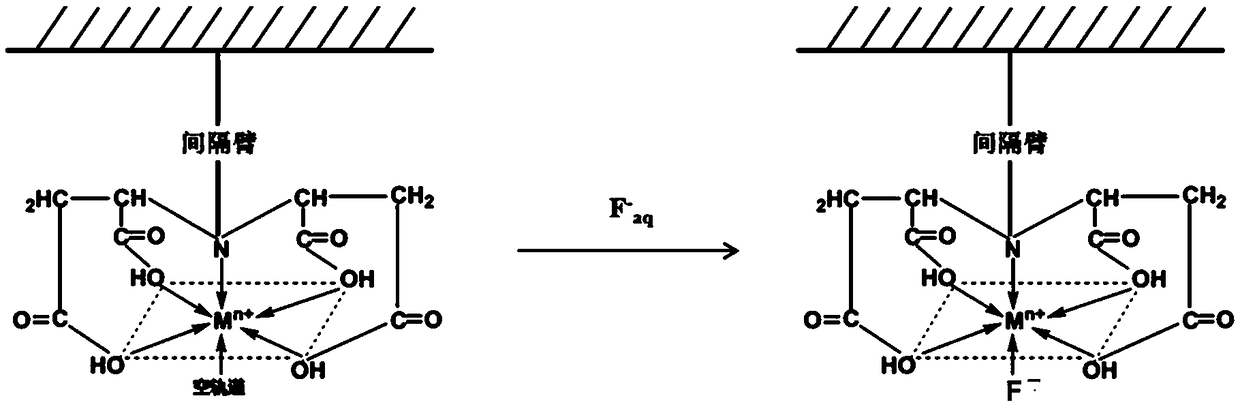
[0044] Example 3IDS-Fe 3+ - Silica metal chelate adsorbent and IDA-Fe 3+ - Silica metal chelate adsorbent, and IDA-Fe 3+ - Styrene divinylbenzene metal chelate adsorbent to conduct comparative experiments on the removal of excess fluoride in the aqueous phase system
[0045] 1. Preparation of fluorine-containing waste water simulated liquid
[0046] Accurately weigh 0.2210g of analytically pure NaF, dry it in an oven at 105°C for 2h, then dissolve and dilute it to 100mL, and store it in a polyethylene bottle. Take 15mL fluoride standard stock solution, dilute it to 1000mL, and store it in a polyethylene bottle. This solution contains 15mg / L of fluorine, and this solution is used as the simulated feed solution of fluorine-containing wastewater.
[0047] 2. IDS-Fe 3+ - Silica metal chelate adsorbent and IDA-Fe 3+ - Silica metal chelate adsorbent, and IDA-Fe 3+ - Static adsorption experiment of styrene divinylbenzene metal chelating adsorbent on excess fluoride in wastewat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com