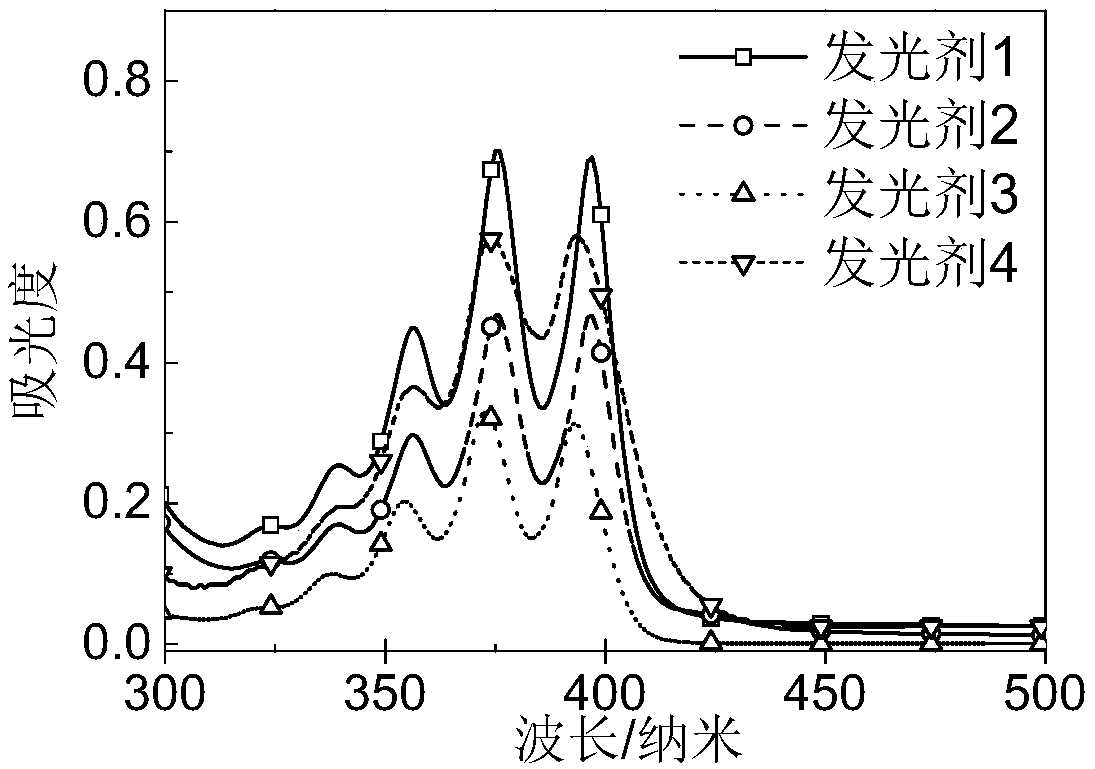
9,10-diphenyl anthracene derivative as reactive luminescent agent, preparation method thereof and high-efficiency weak light up-conversion system prepared from reactive luminescent agent 9,10-diphenyl anthracene derivative
A diphenylanthracene and luminescent agent technology, applied in the field of photon frequency upconversion, can solve the problems of limiting weak light upconversion, unfavorable molecular modification and polymerization, and the upconversion efficiency has not yet exceeded DPA.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Add 2-formylphenylboronic acid (5.63 g, 2.5 equiv, 37.5 mmol) and 9,10-dibromoanthracene (5.05 g, 1 equiv, 15 mmol) in 120 mL toluene and 24 mL ethanol to a 250 mL three-necked flask, and K 2 CO 3 (9.84 g, 69mmol) was dissolved in 48mL of distilled water and mixed into the above solution. Argon was then bubbled into the mixed solution for 15 minutes, followed by the addition of tetrakis(triphenylphosphine) palladium (0) (0.39 g, 1.2 mmol), and then argon was bubbled in for 5 minutes, and heated to reflux in an argon atmosphere to react During the process, the progress of the reaction was tracked by spotting the plate. The developer was dichloromethane / petroleum ether at a ratio of 1:1. After 48 hours, the spot of the raw material 9,10-dibromoanthracene almost disappeared, and the reaction was stopped.
[0058] After the reaction was over, the reaction solution was distilled under reduced pressure to obtain a black solid mixture, and the organic phase was extracted and...
Embodiment 2
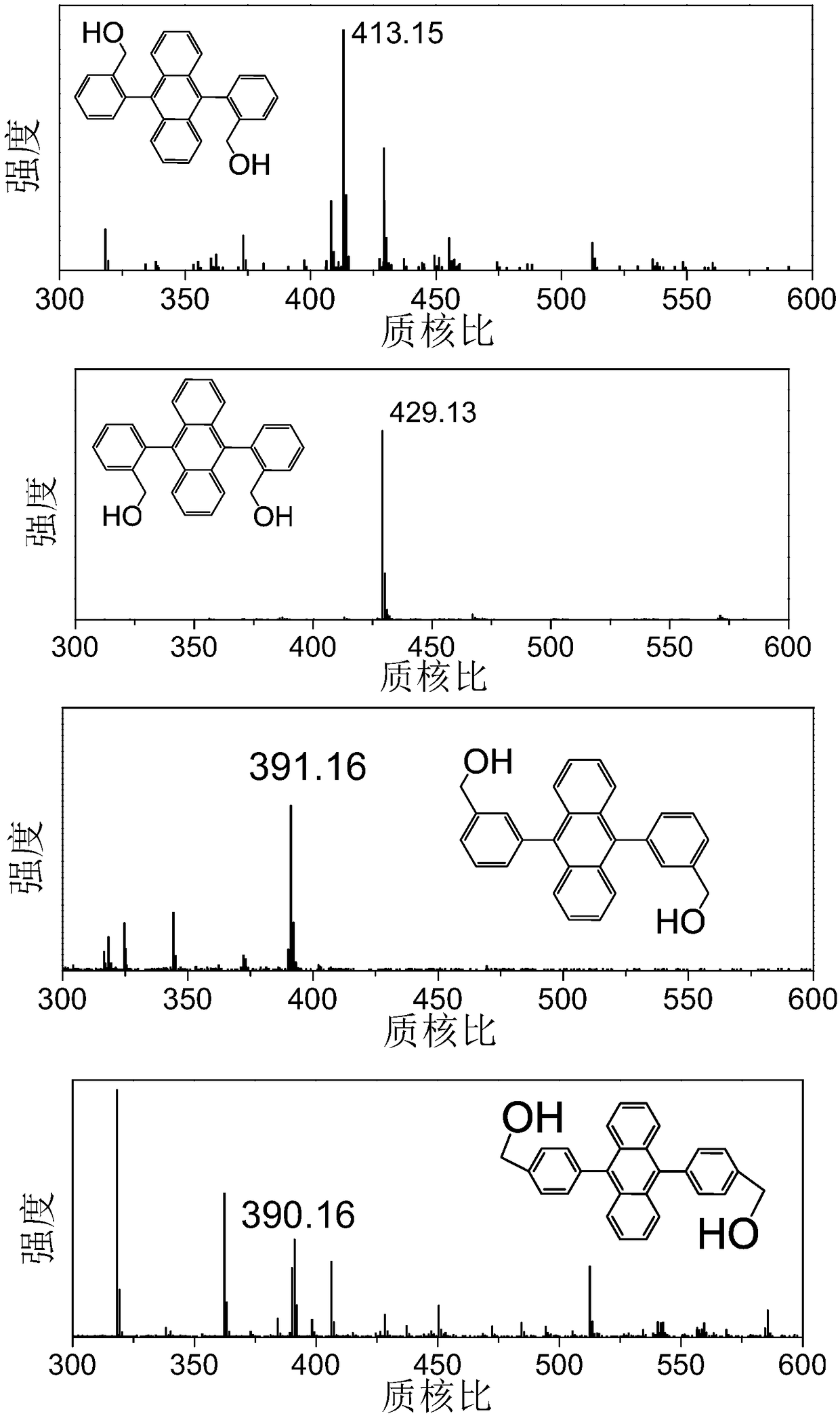
[0065] The reaction solution of Example 1 was distilled under reduced pressure to obtain a black solid mixture, and the organic phase was extracted and separated by dichloromethane and saturated brine several times, and anhydrous Na was added 2 SO 4 After removing water, use column chromatography to separate the product, the developer used is dichloromethane 2:petroleum ether 3, and then undergo secondary purification by recrystallization to obtain a light yellow powder, which is cis 9,10-(2-formyl ) phenylanthracene, hereinafter referred to as cis-o - FDPA, 2.62 g (6.8 mmol), 45.4% yield.
[0066] Melting point: 311.1-312.6°C.
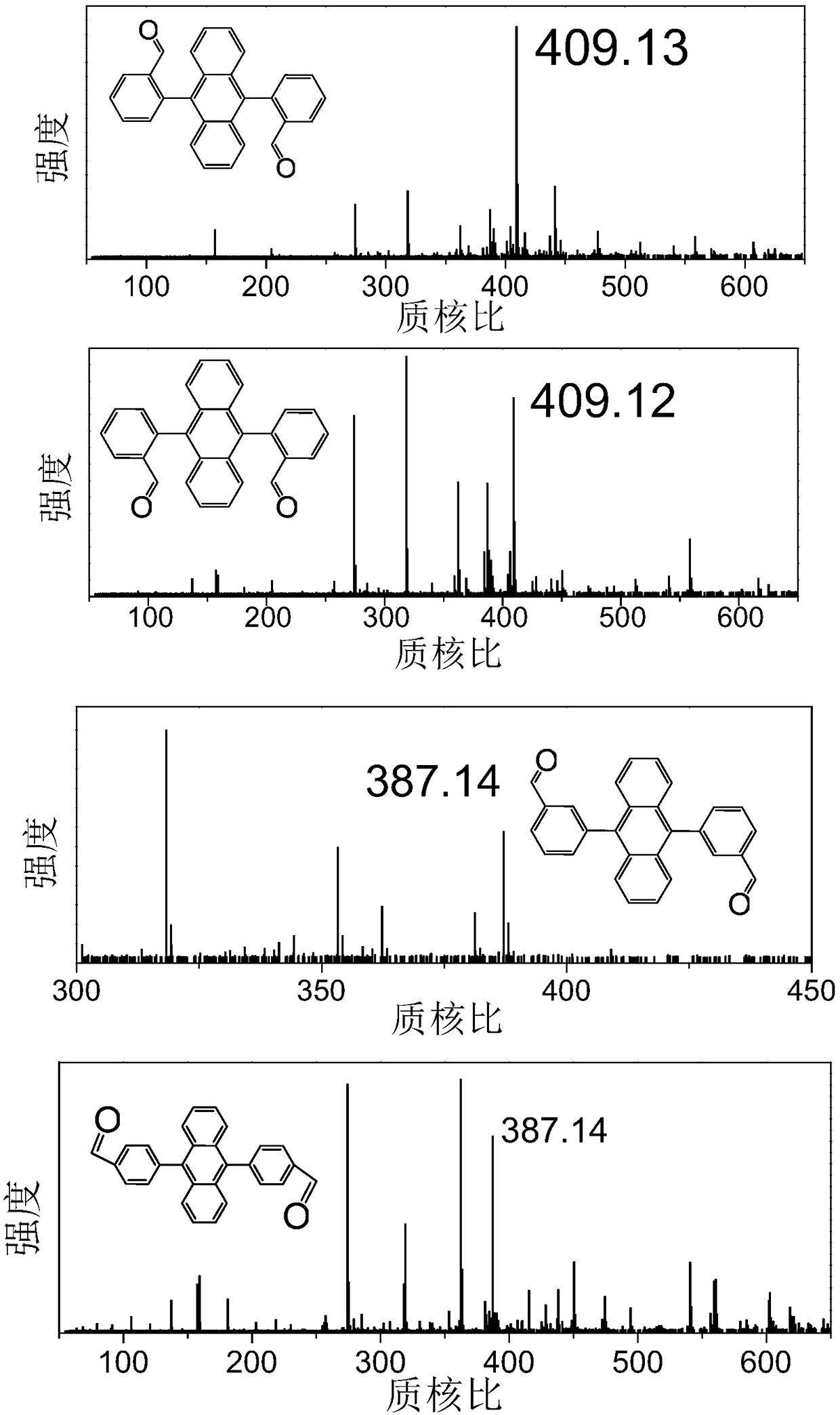
[0067] Mass spectrometry (ESI: m / z ): Calculated 386.13, found 387.14 [M+H] +
[0068] 1 H NMR (400 MHz, DMSO- d 6 ) δ 7.35 – 7.55 (m, 8H), 7.57 – 7.71 (d, J = 7.3Hz, 2H), 7.79 – 7.93 (t, J = 7.4 Hz, 2H), 7.93 – 8.10 (s, 2H), 8.12 – 8.29(d, J = 7.8 Hz, 2H), 9.31 – 9.46 (d, J = 3.3 Hz, 2H).
[0069] The molecular structural formula o...
Embodiment 3
[0072] Add 3-formylphenylboronic acid (2.82 g, 2.5 equiv, 18.8 mmol), 9,10-dibromoanthracene (2.53 g, 1 equiv, 7.5 mmol) in 60 mL toluene and 12 mL ethanol into a 150 mL three-necked flask, and K 2 CO 3 (5.92 g, 34.5 mmol) was dissolved in 23 mL of distilled water and mixed into the above solution. Argon was then bubbled into the mixed solution for 15 minutes, followed by the addition of tetrakis(triphenylphosphine) palladium (0) (0.25 g, 0.82 mmol), and then argon was bubbled in for 5 minutes, then heated to reflux in an argon atmosphere, and the reaction During the process, the progress of the reaction was tracked by spotting the plate. The developer was dichloromethane 3: petroleum ether 4. The reaction lasted for 48 hours, and the spots of the raw material 9,10-dibromoanthracene almost disappeared, so the reaction was stopped.
[0073] After the reaction was over, the reaction solution was distilled under reduced pressure to obtain a black solid mixture, and the organic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com