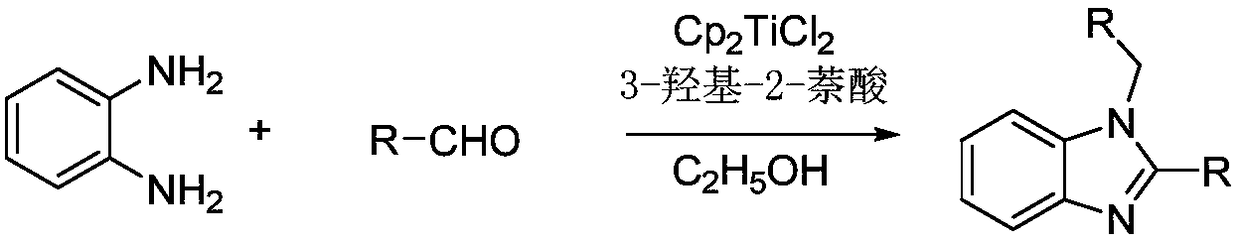
Method for synthesizing 1,2-disubstituted benzimidazole compounds from 3-hydroxy-2-naphthoic acid assisted with titanocene dichloride
A technology of dichlorotitanocene and benzimidazole is applied in the field of synthesis of 1,2-disubstituted benzimidazole compounds, and can solve the problems of difficult preparation of catalysts, tedious post-treatment process, poor reaction selectivity and the like, Achieve the effect of low synthesis cost, simple post-treatment and cheap catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
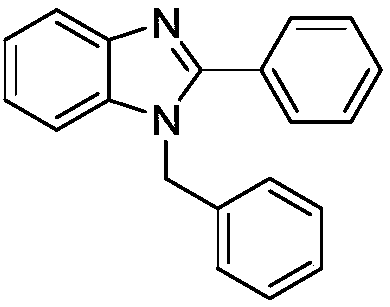
[0013] Synthesis of 1-Benzyl-2-phenyl-(1H)-benzimidazole
[0014]
[0015] Add 0.1081g (1mmol) o-phenylenediamine, 0.0025g (0.01mmol) titanocene dichloride, 224μL (2.2mmol) benzaldehyde, and 2mL ethanol to the reaction flask, stir at 30°C for 20min, and remove the ethanol by rotary evaporation under reduced pressure. Using silica gel as a stationary phase, ethyl acetate and petroleum ether as a developing solvent, 1-benzyl-2-phenyl-(1H)-benzimidazole was obtained by column chromatography with a yield of 95%. The spectral data of the product are: 1 H NMR (400MHz, CDCl 3 )δ:7.89(d,J=8.0Hz,1H),7.70(dd,J=7.6,1.8Hz,2H),7.45(d,J=7.2Hz,3H),7.36-7.28(m,4H), 7.25-7.18(m,2H),7.10(d,J=6.7Hz,2H),5.45(s,2H); 13 C NMR (101MHz, CDCl 3 )δ: 154.30, 143.10, 136.42, 136.09, 130.09, 129.35, 129.18, 128.89, 127.91, 126.88, 126.06, 123.22, 122.88, 119.99, 110.69, 48.49.
Embodiment 2
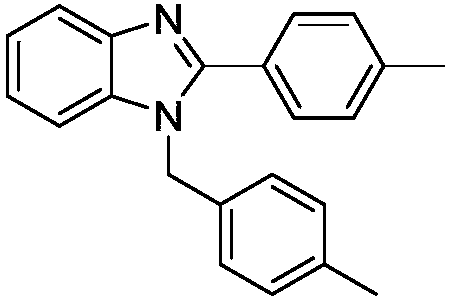
[0017] Synthesis of 1-(4-methylbenzyl)-2-p-tolyl-(1H)-benzimidazole
[0018]
[0019] In this example, the benzaldehyde in Example 1 was replaced with an equimolar amount of p-tolualdehyde, stirred at 40°C for 4 hours, and the other steps were the same as in Example 1 to obtain 1-(4-methylbenzyl)- 2-p-Tolyl-(1H)-benzimidazole, the yield was 80%. The spectral data of the product are: 1 H NMR (400MHz, CDCl 3 )δ: 7.92(d, J=8.0Hz, 1H), 7.64(d, J=7.9Hz, 2H), 7.36-7.29(m, 1H), 7.27(d, J=7.9Hz, 2H), 7.22( q,J=7.5,6.5Hz,2H),7.14(d,J=7.8Hz,2H),7.01(d,J=7.8Hz,2H),5.40(s,2H),2.42(s,3H), 2.35(s,3H); 13 C NMR (101MHz, CDCl 3 )δ: 154.25, 143.20, 139.91, 137.32, 136.09, 133.43, 129.63, 129.38, 129.10, 127.21, 125.84, 122.76, 122.46, 119.77, 110.47, 48.08, 21.36, 21.02.
Embodiment 3
[0021] Synthesis of 1-(4-methoxybenzyl)-2-(4-methoxyphenyl)-(1H)-benzimidazole
[0022]
[0023] In this example, the benzaldehyde in Example 1 was replaced by an equimolar amount of p-methoxybenzaldehyde, stirred at 50°C for 5 hours, and the other steps were the same as in Example 1 to obtain 1-(4-methoxybenzyl )-2-(4-methoxyphenyl)-(1H)-benzimidazole, its productive rate is 96%, and the spectral data of product is: 1 H NMR (400MHz, CDCl 3 )δ: 7.71(d, J=8.0Hz, 1H), 7.50(d, J=8.4Hz, 2H), 7.14(dt, J=7.9, 3.9Hz, 1H), 7.06(d, J=3.7Hz, 2H), 6.87(d, J=8.3Hz, 2H), 6.81(d, J=8.5Hz, 2H), 6.69(d, J=8.4Hz, 2H), 5.20(s, 2H), 3.66(s, 3H), 3.61(s, 3H); 13 C NMR (101MHz, CDCl 3 )δ: 160.83, 159.03, 154.05, 143.08, 136.02, 130.61, 128.37, 127.14, 123.12, 122.69, 122.47, 122.32, 119.56, 114.33, 114.12, 113.99, 110.462, 55.7
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com