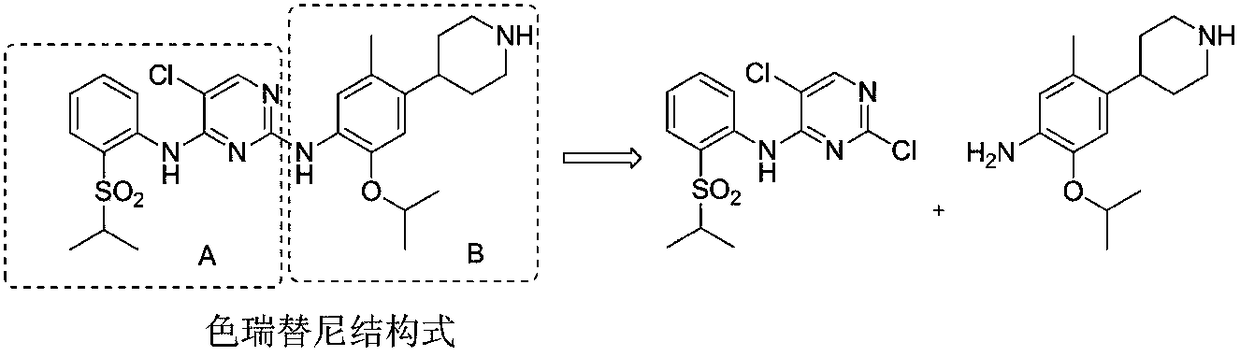
Method for preparing antitumor drug ceritinib intermediate
An anti-tumor drug, ceritinib technology, applied in the field of medicinal chemistry, can solve the problems of difficult recovery of precious metal Pd and organic ligands, high production cost, and achieve the effect of reducing catalytic cost, improving efficiency and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] In order to remove noble metal Pd and phosphorus-containing ligands, the present invention screens metal salts through high-throughput, in order to obtain a simple catalytic system. The present invention screens the following catalysts:
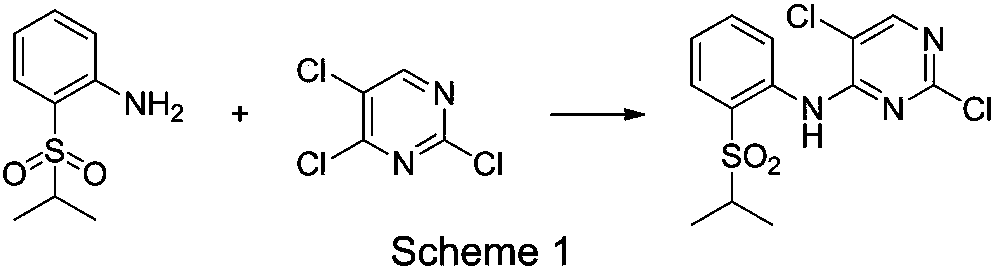
[0031] Add 2-(isopropylsulfonyl)aniline (1.99g, 10mmol), 2,4,5-trichloropyrimidine (2.75g, 15mmol, 1.5eq), DMSO 36ml, potassium tert-butoxide (1.68 g, 15mmol, 1.5eq), metal salt (2mmol, 0.2eq) were reacted at 80-90°C, and the reaction solution was taken every 2h for HPLC analysis, and the 2-(isopropylsulfonyl group in the reaction solution was sampled twice before and after ) When the aniline concentration no longer changes, it is considered as the end of the reaction by default, and the catalytic effects of each metal salt are shown in Table 1:
[0032] The screening of table 1 metal salt catalyst
[0033]
[0034]
[0035] Note: The peak area of 2,4,5-trichloropyrimidine is not counted when the area normalization method is u...
Embodiment 2
[0038] When zinc acetate was determined to be the catalyst, the present invention further optimized the solvent type and reaction temperature, and added 2-(isopropylsulfonyl)aniline (1.99g, 10mmol), 2,4,5-tris Chloropyrimidine (2.75g, 15mmol, 1.5eq), solvent 36ml, potassium tert-butoxide (1.68g, 15mmol, 1.5eq), zinc acetate (2mmol, 0.2eq) were reacted at different temperatures, and the reaction solution was taken every 2h Carry out HPLC analysis, when 2-(isopropylsulfonyl) aniline concentration no longer changes in the two sampling reaction solutions before and after, acquiesce as reaction finishes, count the reaction situation of each reaction system, the result is as shown in table 2:
[0039] Table 2 The optimization of solvent type and reaction temperature
[0040]
[0041]
[0042] The above results show that the use of N-methylpyrrolidone (NMP) and PEG400 as a solvent has achieved a good reaction conversion rate, but due to the high viscosity of PEG400, NMP is sele...
Embodiment 3
[0044]When establishing zinc acetate as a catalyst, NMP as a solvent, and a reaction temperature of 120°C, the molar amount of the present invention to the type of alkali, 2,4,5-trichloropyrimidine (in terms of 2-(isopropylsulfonyl)aniline moles) amount as the benchmark), the catalyst dosage (based on the molar amount of 2-(isopropylsulfonyl) aniline) has been further optimized;
[0045] Add 2-(isopropylsulfonyl)aniline (1.99g, 10mmol), 2,4,5-trichloropyrimidine (11-20mmol, 1.1-2.0eq), NMP 36ml, base (15mmol, 1.5 eq), zinc acetate (0.5-5mmol, 0.05-0.5eq) were reacted at 120°C, and the reaction solution was taken every 2 hours for HPLC analysis, and the concentration of 2-(isopropylsulfonyl)aniline in the reaction solution was sampled twice before and after When it does not change, it is considered as the end of the reaction by default, and the reaction conditions of each reaction system are counted, and the results are shown in Table 3:
[0046] Table 3 The influence of catal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com