Intermediate for preparing bictegravir and preparation method thereof
A technology for intermediates and compounds, which is applied in the preparation of intermediates for bictegravir and the preparation field thereof, can solve the problems of low yield, low epoxidation yield and high cost, achieve high yield, mild route reaction conditions, and improve stereo selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The compound of formula I is condensed with hydroxylamine to obtain the compound of formula II; wherein RCOOH is D-alanine.
[0072]
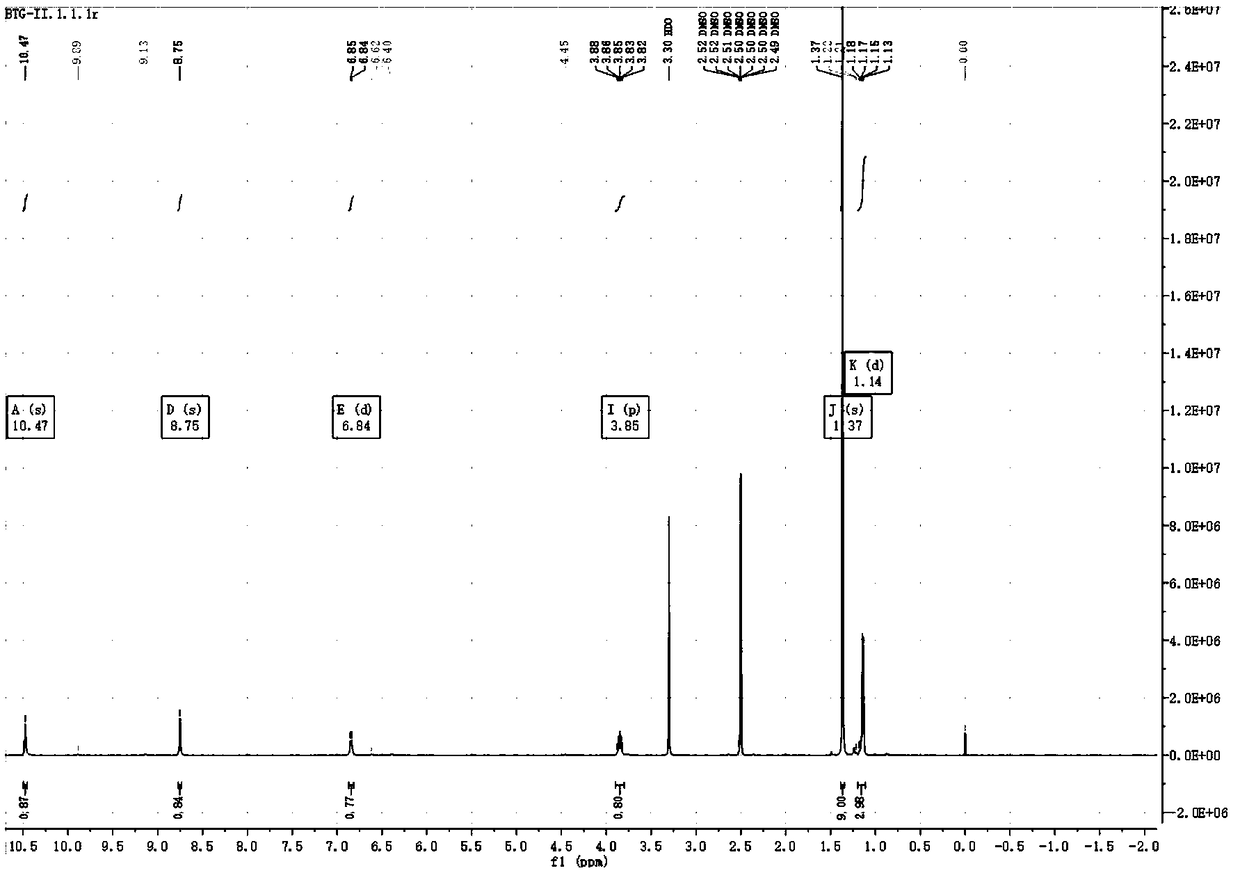
[0073] Dissolve 147.8g of the compound of formula I in 450mL of tetrahydrofuran, add 79.5g of triethylamine, lower the temperature to 0-5°C, add 106.5g of isobutyl chloroformate dropwise, a large amount of solids precipitate out, and keep warm for 2 hours; dissolve 81.75g of hydroxylamine hydrochloride in In 750mL of methanol, cool down to 0-5°C, add sodium hydroxide in batches, a large amount of solids precipitate, keep warm for 2h, add the filtrate from step 1, and continue stirring for 2h. Suction filtration, the filtrate was concentrated under reduced pressure, 1L ethyl acetate was added, a white solid precipitated, suction filtration, the filter cake was washed with 200mL ethyl acetate, and concentrated to dryness. 750mL of dichloromethane was beaten, suction filtered, and air-dried at 45°C to obtain 137.2g of white solid with ...
Embodiment 2
[0075] The compound of formula II is oxidized and asymmetric Diels-Alder reaction to obtain the compound of formula III; wherein RCOOH is D-alanine.
[0076]
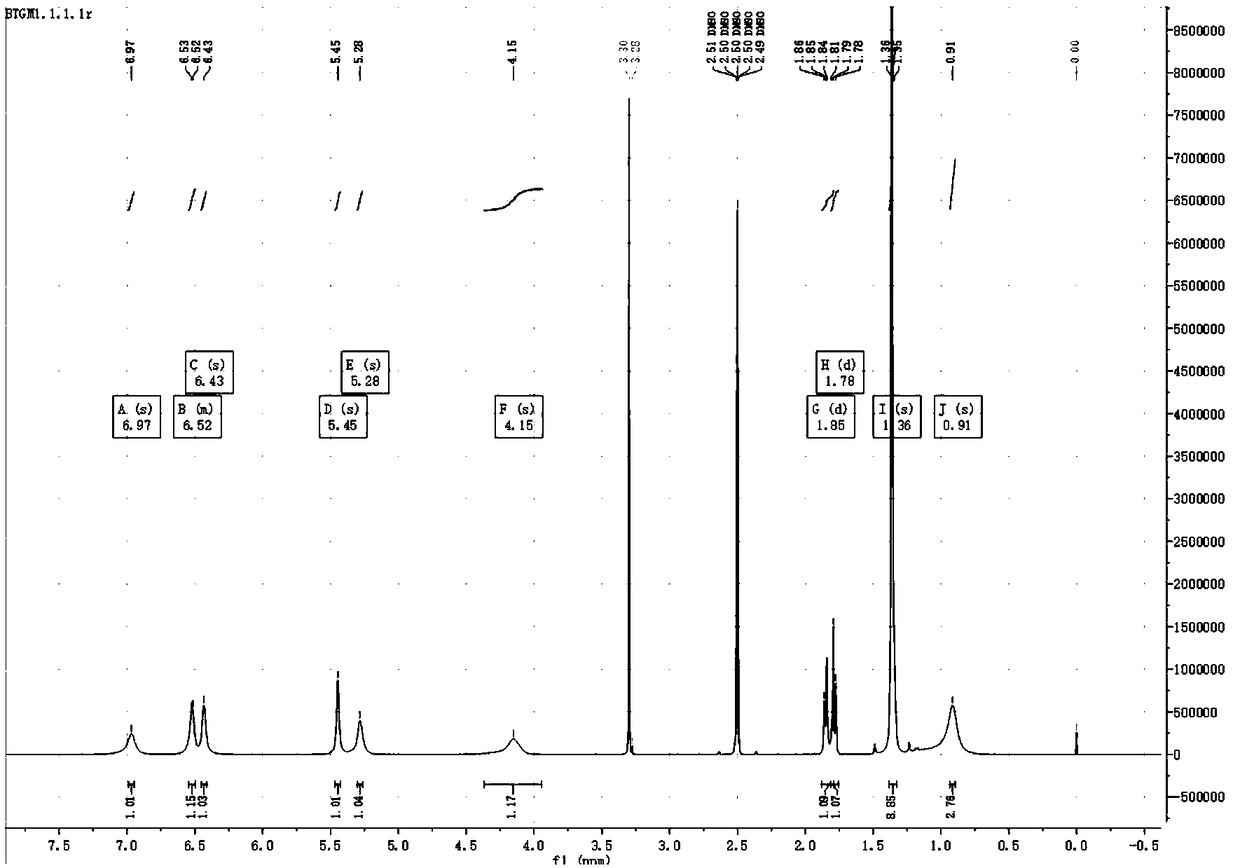
[0077] 12.5 g of the compound of formula II was dissolved in 300 mL of methanol and 100 mL of water, and the temperature was lowered to 0-5°C. Add 12.6g of sodium periodate and 14mL of cyclopentadiene, the color of the solution becomes dark rapidly, a large amount of light yellow solid precipitates, the temperature rises slightly, and the temperature is controlled not to exceed 5°C. After stirring for 30min, add 10mL of cyclopentadiene and 7.6 g sodium periodate, heat preservation reaction 1h. Spin-dried, column chromatography gave 11.9 g of white solid, yield 73%.
[0078] 1 H NMR (500MHz, DMSO-d 6 )δ6.97(s,1H),6.55–6.50(m,1H),6.43(s,1H),5.45(s,1H),5.28(s,1H),4.15(s,1H),1.85(d ,J=9.1Hz,1H),1.78(d,J=8.9Hz,1H),1.36(s,9H),0.91(s,3H).
Embodiment 3
[0080] The compound of formula III obtains the compound of formula IVa through cleavage of the amide bond, and the compound of formula IVa undergoes catalytic hydrogenation and Boc protection to obtain the compound of formula V; wherein RCOOH in the compound of formula III is D-alanine;
[0081]
[0082]
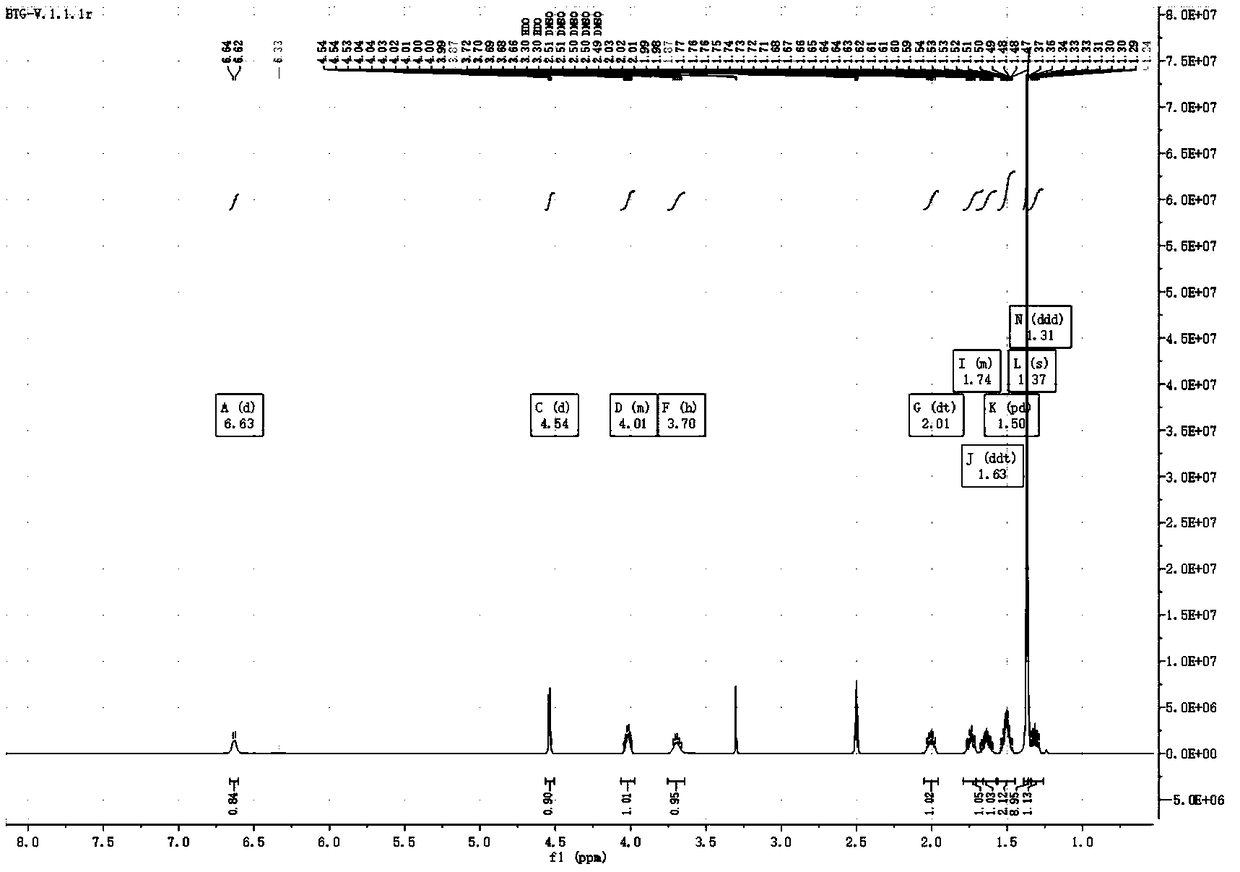
[0083] Dissolve 10g of the compound of formula III in 60mL of methanol and 20mL of water, add 2.5g of lithium hydroxide at room temperature, stir for 2h, add 8.1g of Boc anhydride, and stir for 1h at room temperature. Spin dry, add 20mL water, extract twice with 30mL dichloromethane, and dry over anhydrous sodium sulfate. Spinning to dryness and column chromatography gave 3.6 g of the compound of formula V as an oil, with a yield of 48%.
[0084] 1 H NMR (500MHz, DMSO-d6) δ6.63 (d, J = 7.7Hz, 1H), 4.54 (d, J = 4.5Hz, 1H), 4.06-3.97 (m, 1H), 3.70 (h, J = 7.3Hz, 1H), 2.01(dt, J=13.5, 6.9Hz, 1H), 1.79-1.66(m, 1H), 1.63(ddt, J=13.8, 10.0, 6.5Hz, 1H), 1.50(pd, J =8.5,4.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com