The synthetic method of trimethoxyboroxane
A technology of trimethoxyboron, synthesis method, applied in chemical instruments and methods, compounds containing periodic table Group 3/13 elements, organic chemistry, etc. , three wastes and other problems, to achieve the effect of improving reversible specific capacity, high purity, and improving performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
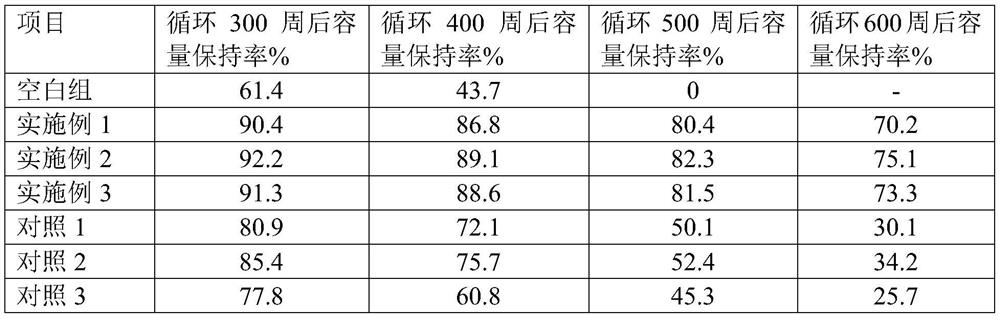
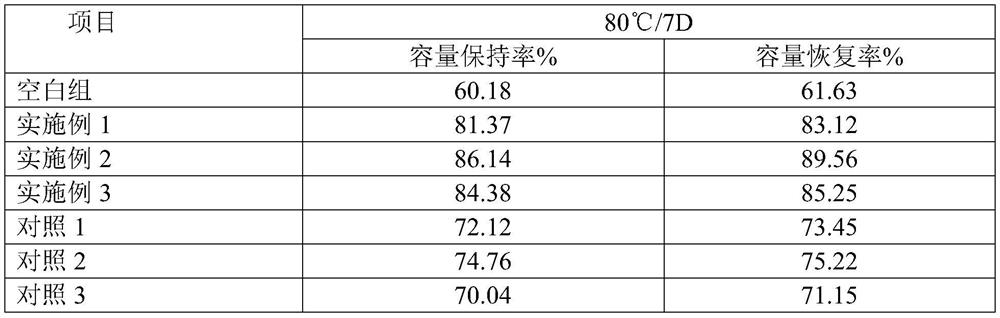
[0017] At -80°C, add 1 mol of borane methyl sulfide into the Schlenk reaction flask, add 6 mol of THF to it, then mix for 20 seconds under a vacuum of 0.7 Pa, then pass carbon dioxide into it, control the pressure at 100 kPa, first in The temperature was raised to 20°C within 15min, then heated at a heating rate of 3°C / min for 10min, then heated at a heating rate of 3°C / min for 10min, and then heated to 80°C, no borane methyl sulfide was detected, and the reaction ended ( After heating up to 80°C, the timed reaction only takes 30 minutes) to obtain trimethoxyboroxane.
[0018] The obtained trimethoxyboroxane density is 1.1g / cm 3 , boiling point 130°C 760mmHg, purity 99.56%, acid value 34ppm, water content 28ppm, yield 95.3%.
Embodiment 2
[0020] At -70°C, add 1 mol of borane methyl sulfide into the Schlenk reaction flask, add 8 mol of THF to it, then mix for 30 seconds under a vacuum of 0.5 Pa, then pass carbon dioxide into it, control the pressure at 90 kPa, first in Heat up to 30°C within 15min, then heat at a heating rate of 1.5°C / min for 15min, then heat at a heating rate of 2°C / min for 10min, heat up to 72.5°C, detect no borane methyl sulfide, and the reaction ends ( After heating up to 72.5°C, the timed reaction only takes 40 minutes) to obtain trimethoxyboroxane.
[0021] The obtained trimethoxyboroxane density is 1.15g / cm 3 , boiling point 131°C 760mmHg, purity 99.68%, acid value 32ppm, water content 25ppm, yield 95.8%.
Embodiment 3
[0023] At -60°C, add 1 mol of borane methyl sulfide into the Schlenk reaction flask, add 7 mol of THF to it, then mix for 25 seconds under a vacuum of 0.6 Pa, then pass carbon dioxide into it, control the pressure at 95 kPa, first in Heat up to 25°C within 15min, then heat at a heating rate of 2°C / min for 10min, then heat at a heating rate of 2°C / min for 15min, then heat up to 75°C, detect no borane methyl sulfide, and the reaction ends ( After heating up to 75°C, the timed reaction only takes 35 minutes) to obtain trimethoxyboroxane.
[0024] The obtained trimethoxyboroxane density is 1.15g / cm 3 , boiling point 129°C 760mmHg, purity 99.62%, acid value 35ppm, water content 30ppm, yield 96.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com