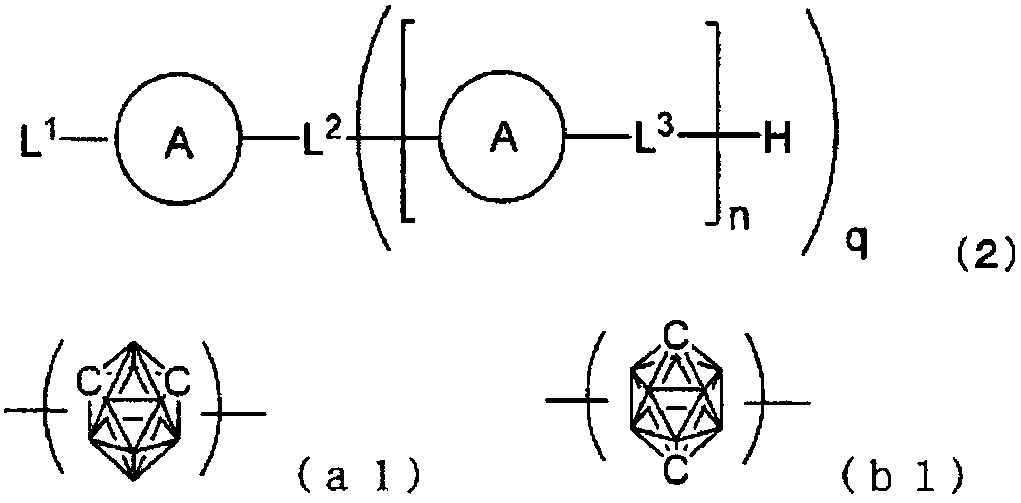Organic electroluminescent element
A luminescent, organic field technology, applied in the field of organic electroluminescent components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
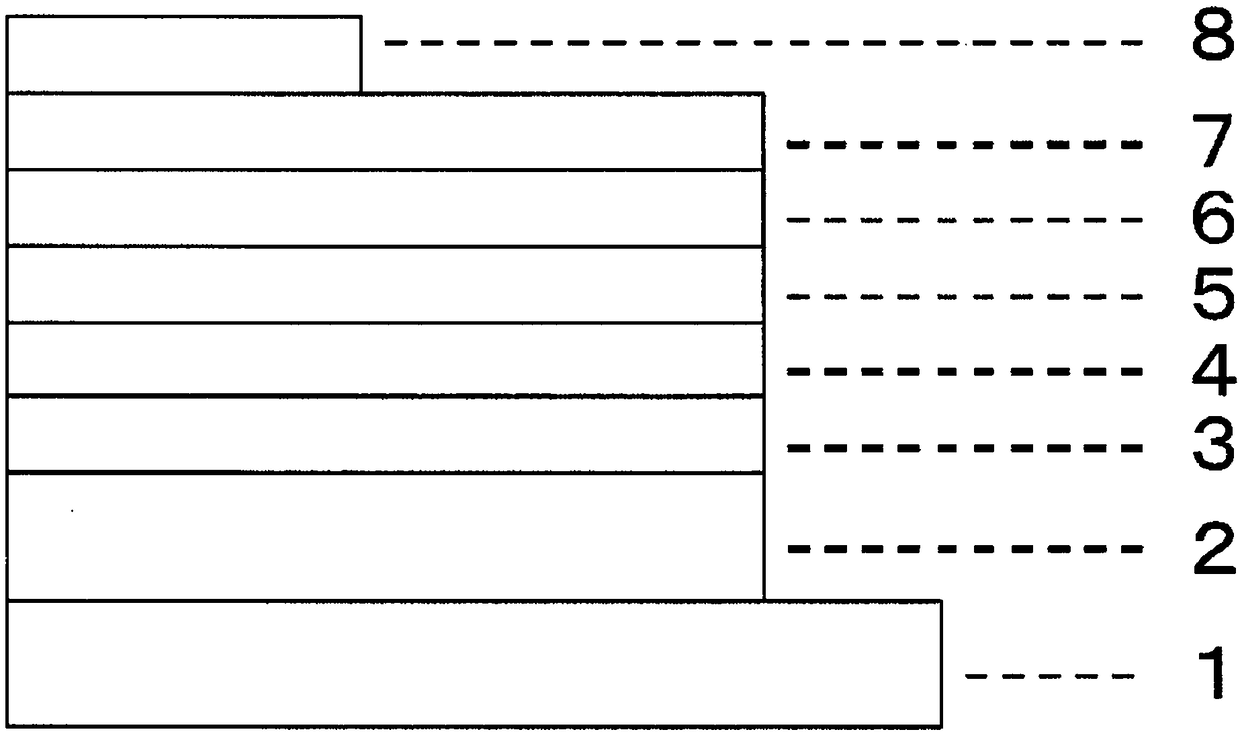
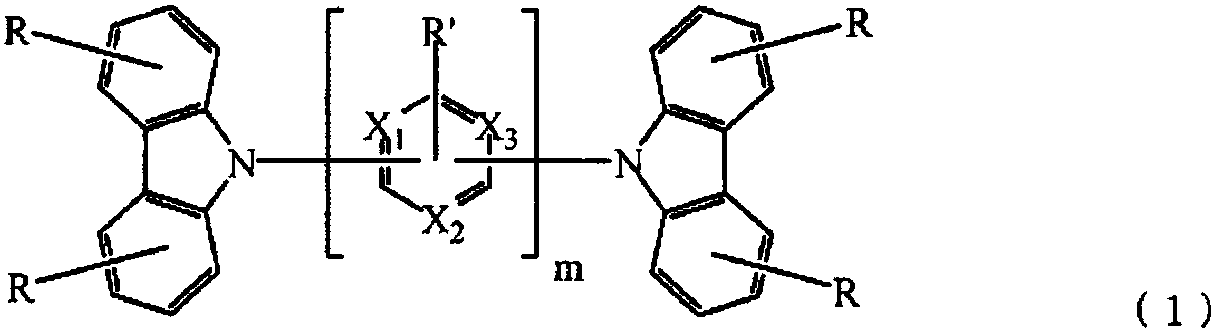
[0136]On a glass substrate on which an anode made of indium tin oxide (ITO) with a film thickness of 70 nm was formed, a vacuum deposition method was used to obtain a vacuum of 2.0×10 -5 Pa laminates each film. First, on ITO, copper phthalocyanine (CuPC) was formed to a thickness of 30 nm as a hole injection layer. Next, 4,4-bis[N-(1-naphthyl)-N-phenylamino]biphenyl (NPB) was formed as a hole transport layer with a thickness of 15 nm. Next, as the light-emitting layer, compound 1-2 as the first host, compound 2-1 as the second host, and iridium complex [bis(4,6-difluorobenzene] which is a blue phosphorescent material as the guest of the light-emitting layer base)-pyridine-N,C2']iridium picolinate(III)](FIrpic) was co-evaporated from different evaporation sources to form a light-emitting layer with a thickness of 30 nm. At this time, the vapor deposition rate ratio (wt ratio) of the first body, the second body, and FIrpic was 47:47:6. Next, Alq is formed with a thickness of ...
Embodiment 2~21
[0139] In Example 1, organic EL elements (Examples 2 to 7) were produced in the same manner as in Example 1, except that the compounds described in Table 1 were used as the first host of the light-emitting layer.
[0140] In addition, organic EL elements (Examples 8 to 21) were produced in the same manner as in Examples 1 to 7, except that Compounds 2-18 and 2-29 were used as the second host of the light-emitting layer.
[0141] When an external power source was connected to the obtained organic EL element and a DC voltage was applied, an emission spectrum with a maximum wavelength of 475 nm was observed from any organic EL element, and it was found that light emission from FIrpic was obtained. Table 1 shows the characteristics of the produced organic EL elements.
Embodiment 22
[0151] On a glass substrate on which an anode made of ITO with a film thickness of 150 nm was formed, a vacuum degree of 4.0×10 was carried out by a vacuum deposition method. -4 Pa laminates each film. First, CuPc was formed as a hole injection layer with a thickness of 20 nm on ITO, and then NPB was formed as a hole transport layer with a thickness of 20 nm. Next, as the light-emitting layer, compound 1-2 as the first host, compound 2-1 as the second host, and tris(2-phenylpyridine)iridium(III)(Ir(PPy) as the guest of the light-emitting layer 3 ) were co-evaporated from different evaporation sources, and formed with a thickness of 30 nm. At this time, the first body, the second body and Ir(PPy) 3 The evaporation rate ratio of 47:47:6. Next, bis(2-methyl-8-hydroxyquinoline)4-phenylphenolaluminum(III)(BAlq) was formed as a hole blocking layer with a thickness of 10 nm. Next, Alq was formed with a thickness of 40 nm 3 as an electron transport layer. Further, LiF was formed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com