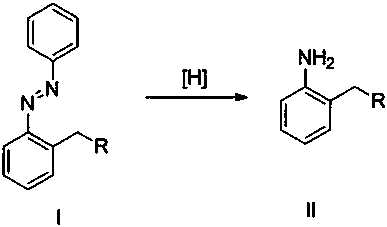
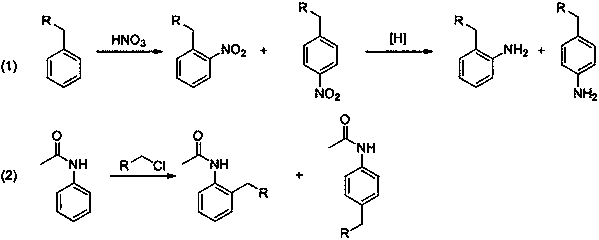
Synthetic method for ortho-alkylaniline
A technology of an alkylaniline and a synthesis method, applied in the field of chemistry, can solve the problems of waste of raw materials, influence on the synthesis efficiency of ortho-position alkylaniline compounds, by-products, etc., and achieves low side reactions, high product yield and easy realization. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
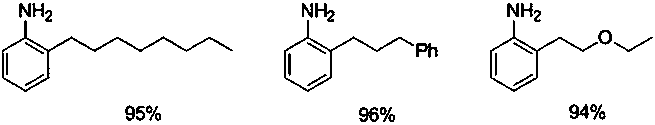
[0014] Add 294 mg (1 mmol) of 2-heptylazobenzene, 224 mg (4 mmol) of iron powder, 0.5 mL of acetic acid, and 20 mL of ethanol into the reaction tube. Under nitrogen, stir at room temperature for 24 hours. After the reaction, separate by column chromatography to obtain The target product 2-heptylaniline was 195 mg, and the yield was 95%.
Embodiment 2
[0016] Add 300 mg (1 mmol) of 2-(3-phenylpropyl)nitrobenzene, 224 mg (4 mmol) of iron powder, 0.5 mL of acetic acid, and 20 mL of ethanol to the reaction tube, and stir at room temperature for 24 hours under nitrogen. After the reaction, the column After chromatographic separation, 202 mg of the target product 2-(3-phenylpropyl)aniline was obtained with a yield of 96%.
Embodiment 3
[0018] Add 254 mg (1 mmol) of 2-(2-ethoxyethyl) azobenzene, 260 mg (4 mmol) of zinc powder, 0.5 mL of hydrochloric acid, and 20 mL of ethanol into the reaction tube, stir at room temperature for 24 hours under nitrogen, and react Afterwards, column chromatography separated to obtain the target product 2-(2-ethoxyethyl)aniline 155mg, and the yield was 94%.
[0019] Add 294mg (1 mmol) of 2-heptyl azobenzene in the reaction kettle, Pa / C catalyst 33mg, methanol 25mL, nitrogen-hydrogen replacement, under 1MPa pressure, 80 o C was stirred for 10 hours, and after the reaction, column chromatography separated to obtain 195 mg of 2-heptylaniline, and the yield was 95%.
[0020] Following is the synthetic product and corresponding productive rate adopting the technical scheme of the present invention:
[0021]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com