Preparation method and application of bilastine
A technology for bilastine and bilastine methyl ester, which is applied in the field of organic compound synthesis, can solve problems such as difficult industrialized production, complex synthesis route, potential safety hazard, etc., and achieves reduction of operation steps, shortening of synthesis route, and reduction of industrialized operations. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
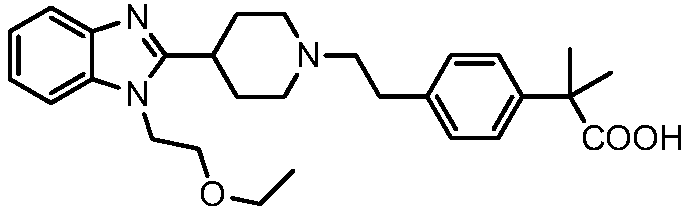
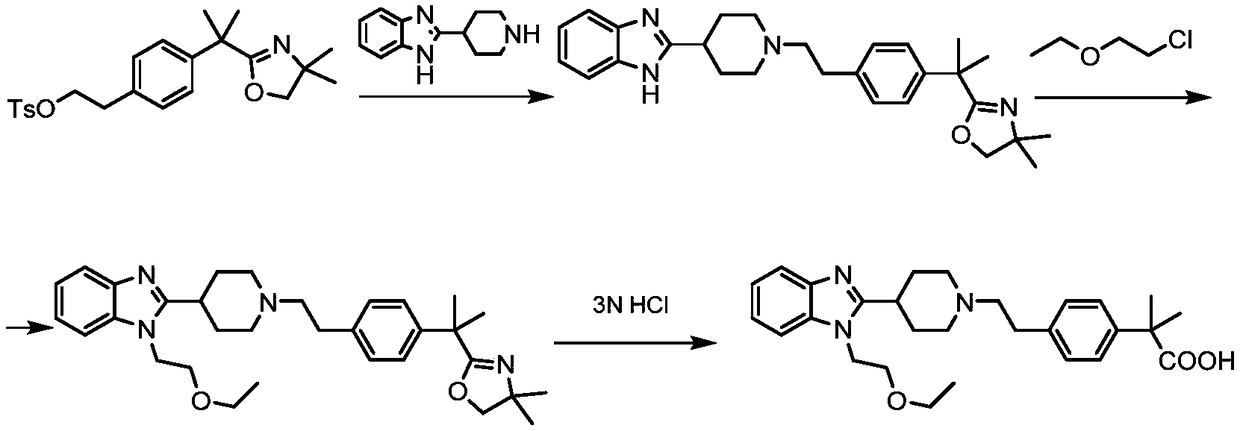
[0049] A preparation method of bilastine, the preparation route is as follows:
[0050]
[0051]The synthetic route of bilastine provided by the present invention shortens the synthetic route, makes the crude product of bilastine produced contain less impurities, is convenient for refining, and improves the yield of final refining, thereby reducing industrial operations and reducing cost.
[0052] The preparation method of described bilastine specifically comprises the following steps:
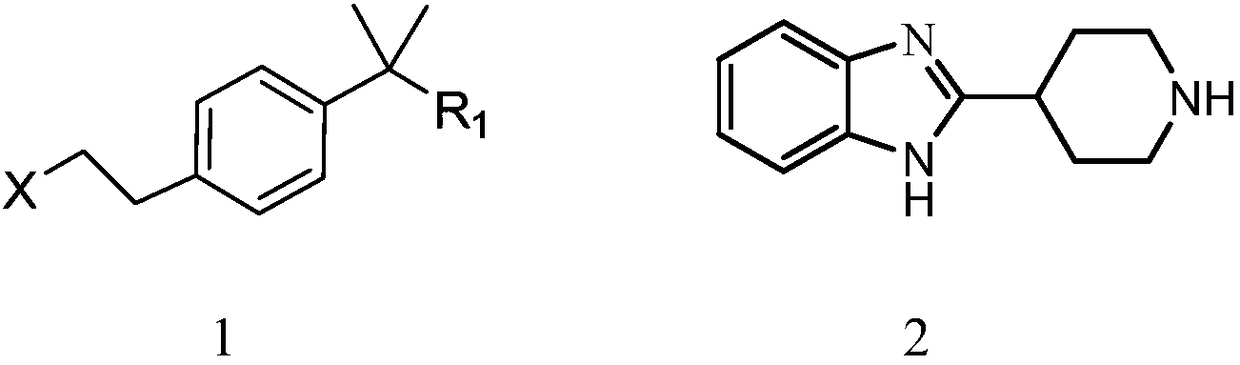
[0053] Using 2-methyl-2-{4-[2-(toluene-4-sulfonyloxy)-ethyl]-phenyl}-propionic acid methyl ester as starting material and 1-(2-ethoxy -Ethyl)-2-piperidin-4-yl-1H-benzimidazole is condensed into bilastine methyl ester, bilastine methyl ester is hydrolyzed into bilastine crude product in alkaline medium, and bilastine methyl ester is hydrolyzed into bilastine crude product, Rastin crude refined to get bilastine.
[0054] Preferably, the preparation method of the 2-methyl-2-{4-[2-(toluene-4...
Embodiment 1
[0079] (1) preparation of bilastine methyl ester
[0080] Dissolve 1-(2-ethoxy-ethyl)-2-piperidin-4-yl-1H-benzimidazole (27.34g, 0.1mol) and diisopropylethylamine (39g, 0.3mol) in In tetrahydrofuran (200ml), slowly add 2-methyl-2-{4-[2-(toluene-4-sulfonyloxy)-ethyl]-phenyl}-propionic acid methyl ester (28g, 0.1 mol) in tetrahydrofuran (100ml). After the addition, heat up to reflux and keep warm for 8 hours, add 100ml of water to quench the reaction, then concentrate the reaction solution, extract the concentrated reaction solution with 200ml ethyl acetate for 3 times each time, and wash the combined ethyl acetate layer with water and saturated saline Twice, dried and concentrated to give bilastine methyl ester (39.45 g, 82.6%) as a white solid.
[0081] (2) preparation of bilastine crude product
[0082] Add the bilastine methyl ester (23.88g, 0.05mol) obtained above into methanol (75ml), then add a 30% aqueous solution containing sodium hydroxide (3g, 0.075mol), and cool t...
Embodiment 2
[0086] (1) preparation of bilastine methyl ester
[0087] Dissolve 1-(2-ethoxy-ethyl)-2-piperidin-4-yl-1H-benzimidazole (27.34g, 0.1mol) and diisopropylethylamine (39g, 0.3mol) in In isopropyl ether (200ml), slowly add 2-methyl-2-{4-[2-(toluene-4-sulfonyloxy)-ethyl]-phenyl}-propionic acid methyl ester (28g , 0.1mol) in isopropyl ether solution (100ml). After the addition, heat up to reflux and keep warm for 6 hours, then add 100ml of water to quench the reaction, then concentrate the reaction solution, extract the concentrated reaction solution with 200ml ethyl acetate for 3 times each time, and wash the combined ethyl acetate layer with water and saturated saline Twice, dried and concentrated to give bilastine methyl ester (36.87 g, 77.2%) as a white solid.
[0088] (2) preparation of bilastine crude product
[0089] Add the bilastine methyl ester (23.88g, 0.05mol) obtained above into ethanol (75ml), then add a 30% aqueous solution containing sodium hydroxide (3g, 0.075mol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com