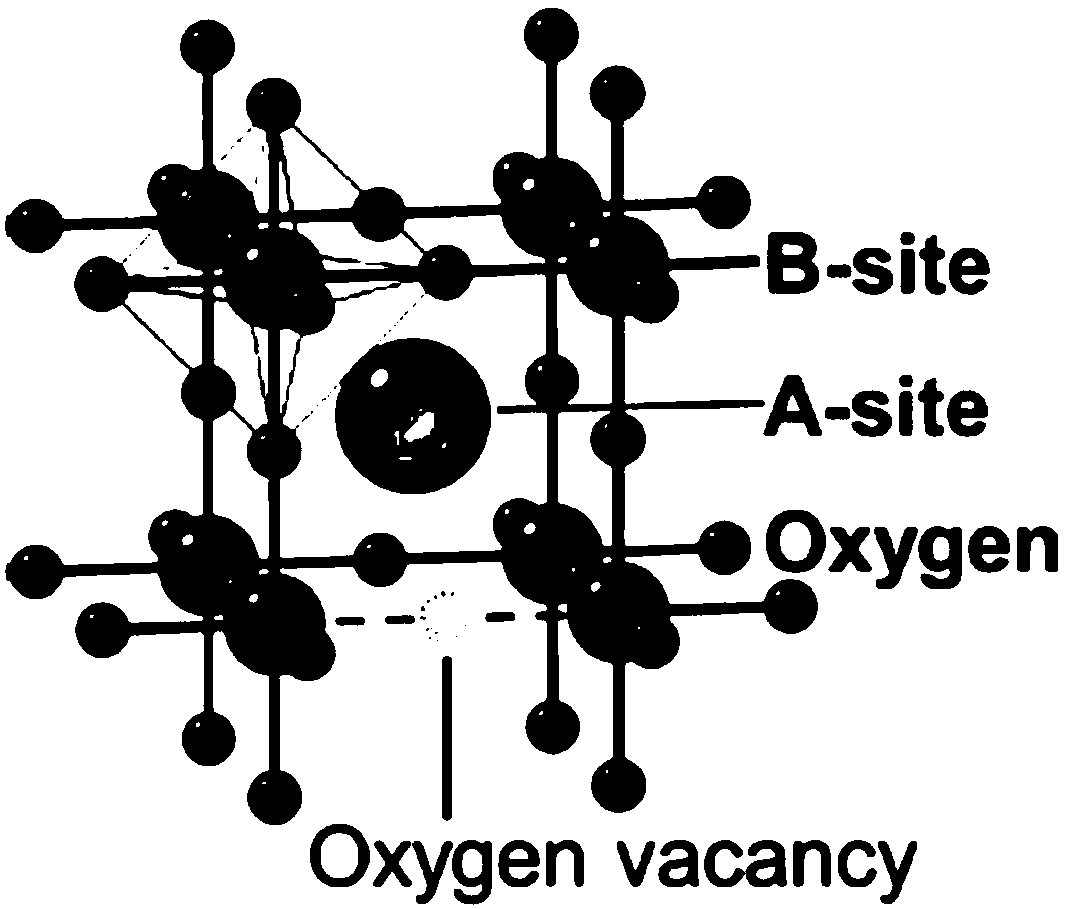
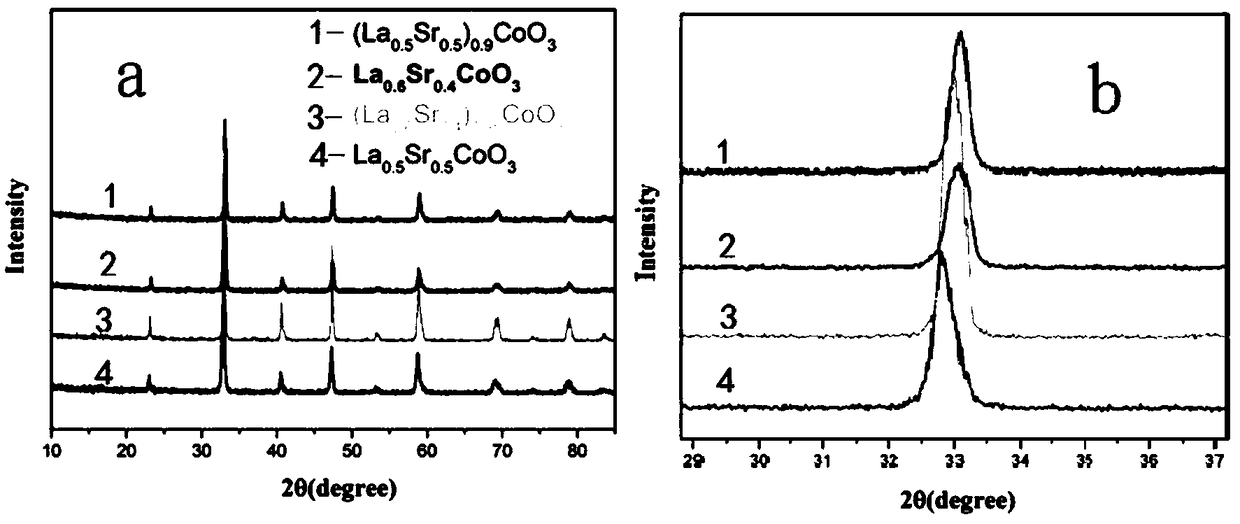
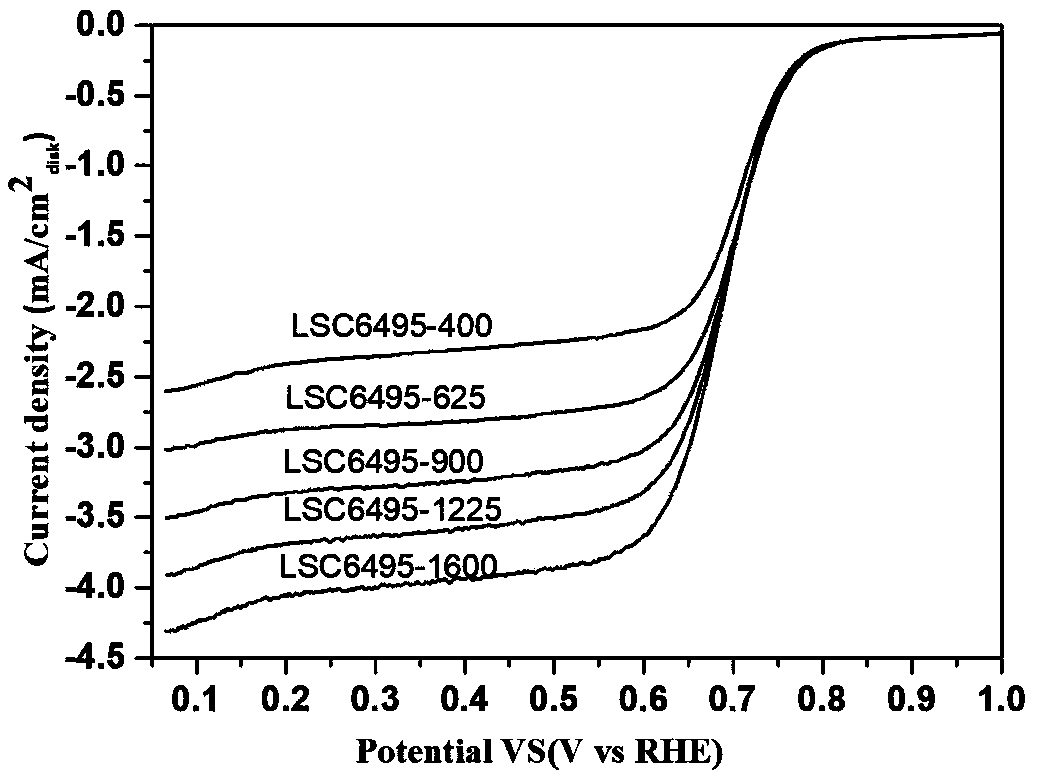
A A-site vacancy type perovskite oxygen catalyst, a preparation method and application thereof
An oxygen catalyst and perovskite technology, which is applied in the field of A-site-deficient perovskite oxygen catalyst and its preparation, can solve the problems of reducing fuel cell production and use costs, low catalytic activity, poor stability, etc., and reach the limit of improvement High current density, high catalytic activity, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]Weigh 5.76 g of lanthanum nitrate, 1.87 g of strontium nitrate, and 6.46 g of cobalt nitrate according to the stoichiometric ratio La:Sr:Co=0.6:0.4:1, and dissolve them in 300 mL of deionized water. Add complexing agent citric acid 17.06g, EDTA12.975g to the solution according to the ratio of total moles of metal ions: citric acid moles: EDTA moles=1:2:1, then add ammonia water to adjust the pH value=7 of the solution, Finally heated to 80°C with magnetic stirring for 10h to obtain La 0.6 Sr 0.4 CoO 3 Precursor gel.
[0032] The obtained precursor gel was dried in an oven at 150°C for 10 hours, and the gel-like liquid gradually turned into a fluffy black solid. Grinding the black solid into a powder in a mortar gave La 0.6 Sr 0.4 CoO 3 Precursor powder.
[0033] The prepared black precursor powder sample was placed in a 100mL corundum crucible, raised from room temperature to 500°C at a rate of 2°C / min, and kept at 500°C for 2 hours to remove excess citric acid an...
Embodiment 2
[0035] Weigh 5.62 g of lanthanum nitrate, 1.83 g of strontium nitrate, and 6.635 g of cobalt nitrate according to the stoichiometric ratio La:Sr:Co=0.57:0.38:1, and dissolve them in 300 mL of deionized water. Add complexing agent citric acid 17.06g, EDTA12.99g to the solution according to the ratio of total moles of metal ions: citric acid moles: EDTA moles=1:2:1, then add ammonia water to adjust the pH value of the solution=7, Finally, it was heated to 80° C. and magnetically stirred for 10 h to obtain a precursor gel.
[0036] The above precursor gel was dried in an oven at 150°C for 10 hours, and the gel-like liquid gradually turned into a fluffy black solid. Grinding the black solid into a powder in a mortar gave (La 0.6 Sr 0.4 ) 0.95 CoO 3 Precursor powder.
[0037] Put the black precursor powder sample in a 100mL corundum crucible, raise the temperature from room temperature to 500°C at a rate of 2°C / min, and keep it at 500°C for 2 hours; then continue to heat up fr...
Embodiment 3
[0040] Weigh 4.91 g of lanthanum nitrate, 2.40 g of strontium nitrate, and 6.60 g of cobalt nitrate according to the stoichiometric ratio La:Sr:Co=0.5:0.5:1, and dissolve them in 300 mL of deionized water. Add complexing agent citric acid 17.45g, EDTA13.27g according to the ratio of total moles of metal ions: moles of citric acid: moles of EDTA=1:2:1, then add ammonia water to adjust the pH value of the solution=7, and finally heat to Magnetic stirring at 80° C. for 10 h to obtain a precursor gel.
[0041] The above precursor gel was dried in an oven at 150°C for 10 hours, and the gel-like liquid gradually turned into a fluffy black solid. Grinding the black solid into a powder in a mortar gave La 0.5 Sr 0.5 CoO 3 Precursor powder.
[0042] Place the prepared black precursor powder sample in a 100mL corundum crucible, raise the temperature from room temperature to 500°C at a rate of 2°C / min, and keep it at 500°C for 2 hours; then continue to heat up from 500°C to 1000°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com