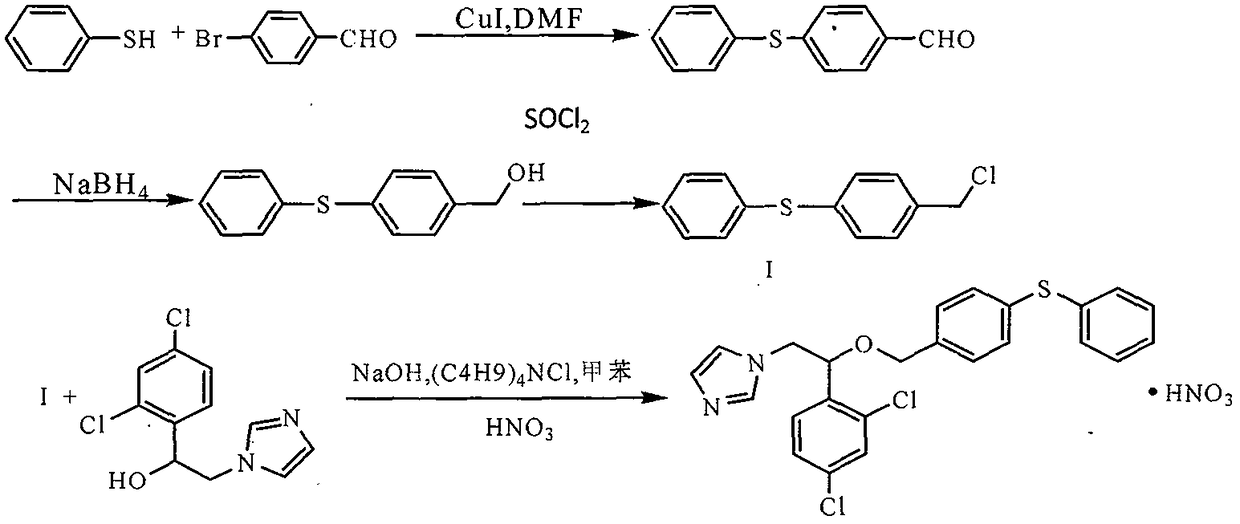
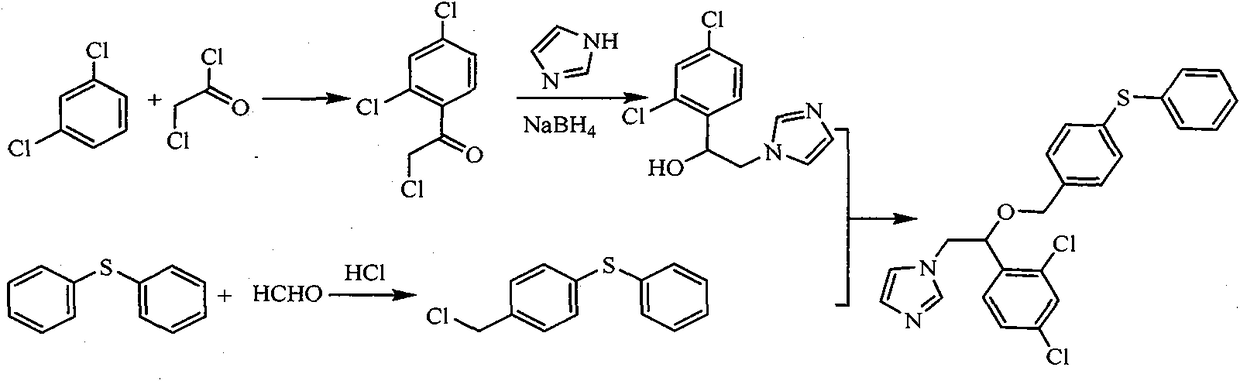
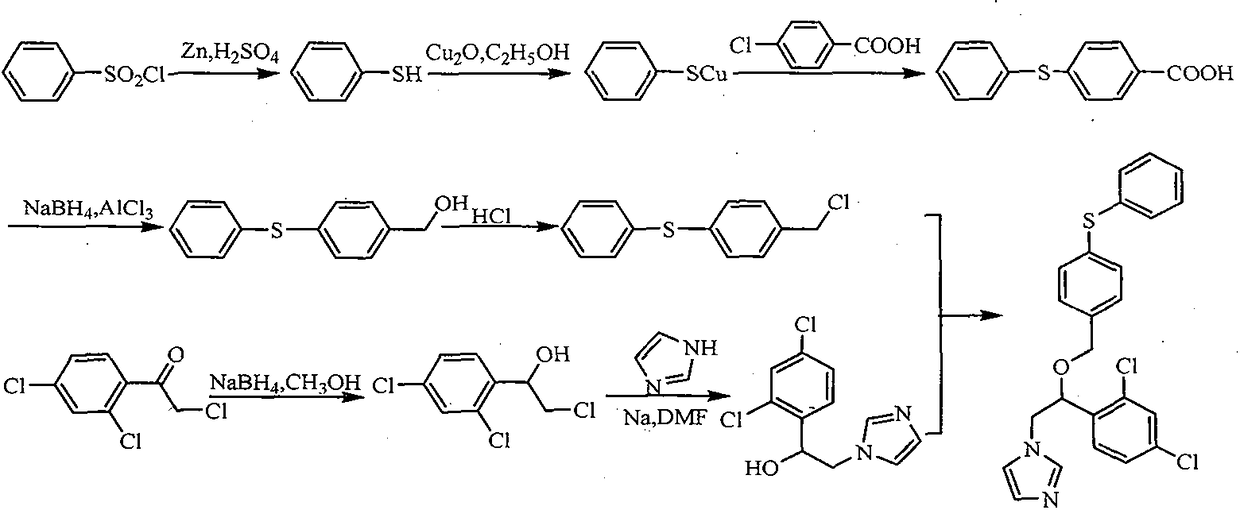
Synthesis process of fenticonazole nitrate
A technology of fenticonazole nitrate and synthesis process, which is applied in the field of medicine and can solve problems such as strong corrosion, high chemical reactivity, and unsuitability for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The synthesis process of fenticonazole nitrate uses cheap and easy-to-obtain reagents, greatly reduces the synthesis cost, is easy to operate, has no special requirements for equipment, and is more suitable for large-scale production. For specific synthesis routes, see figure 1 .
Embodiment 2
[0041] Intermediate I: Synthesis of 4-phenylmercaptobenzaldehyde
[0042] At room temperature (25°C), put 592g of p-bromobenzaldehyde, 360g of thiophenol, 480g of potassium carbonate, 16g of CuI and 2000ml of DMF into a 5000mL reaction flask, stir and heat up to 124-128°C, react for about 6 hours, and perform TLC detection ( n-Hexane: ethyl acetate = 10:1) to the end of the reaction, drop to 90°C and filter, reduce pressure (pressure 1720Pa, temperature 85°C) and concentrate to dryness, recover DMF to obtain a viscous residue, add dichloromethane 2400ml , washed three times with water, dried over anhydrous magnesium sulfate, filtered, the filtrate was concentrated to dryness under reduced pressure, and dichloromethane was recovered, and the residue was dissolved in 240ml of a mixed solution (hexane:ethyl acetate=1:1), frozen and crystallized, filtered, The filter cake was dried under normal pressure at 25° C. to obtain 536 g of white crystalline powder with a yield of 78.3%. ...
Embodiment 3
[0044] Intermediate II: Synthesis of 4-Phenylmercaptobenzyl Alcohol
[0045] At room temperature (25°C), put 527g of 4-phenylmercaptobenzaldehyde and 2965ml of methanol into a 5000mL reaction flask, add 29.65g of sodium borohydride in three batches, stir and raise the temperature to 48°C, and react for about 1 hour. alkane: ethyl acetate = 10:1) to the end of the reaction, hydrochloric acid was added dropwise to adjust the pH to 6, and concentrated to dryness under reduced pressure (pressure 1330Pa, temperature 45°C) to recover methanol. To obtain a viscous residue, add 2200ml of dichloromethane, wash with water until neutral, dry over anhydrous magnesium sulfate, filter, concentrate the filtrate to dryness under reduced pressure (pressure 1720Pa, temperature 25°C), recover dichloromethane, and obtain light yellow crystals Sexual powder 518.7g, yield 97.3%, HPLC purity: 99.56%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com