Detection kit for HPIV (human parainfluenza virus) triple nucleic acid
A technology of human parainfluenza virus and detection kit, which is applied in the field of nucleic acid detection, can solve the problems of poor specificity and low sensitivity of the kit, and achieve the effect of fast operation, high sensitivity and low resolution efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
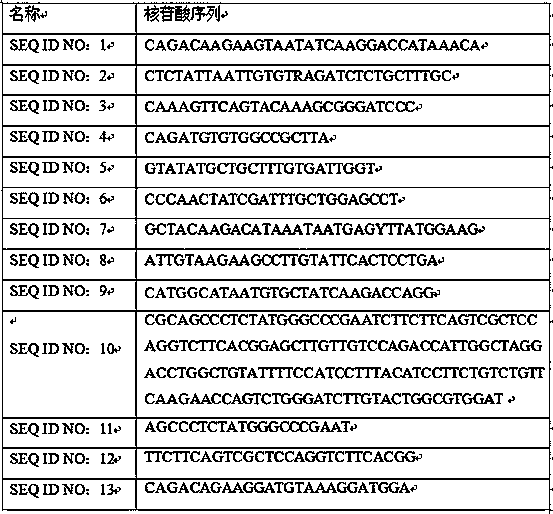
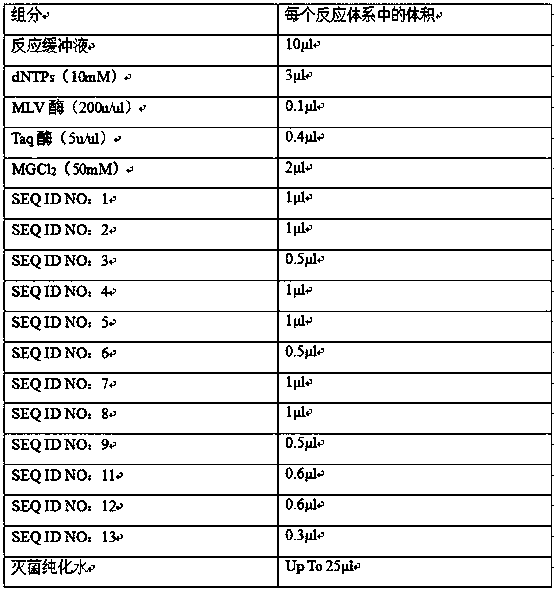
[0017] Example 1 Preparation of Human Parainfluenza Virus Triple Nucleic Acid Detection Kit
[0018] Prepare the PCR reaction system according to the ratio in Table 2
[0019] Table 2
[0020]
[0021] This kit also includes a negative control (sterile water) and a positive control (artificially synthesized at a concentration of 1×10 5 Copies / ml of pseudovirus).
Embodiment 2
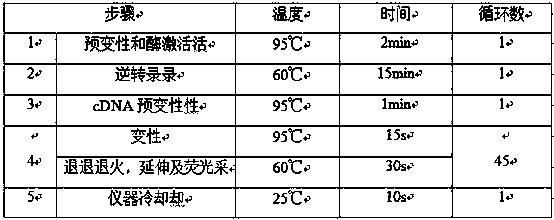
[0022] Embodiment 2 The detection method of kit of the present invention
[0023] The detection method of the present invention is Real time RT-PCR, which combines RNA reverse transcription and DNA amplification. The Real TimeRT-PCR reaction process is (1) cDNA synthesis; (2) pre-denaturation, the time and length depend on the length and base composition of the target nucleotide, the pre-denaturation temperature is generally 90°C-105°C, and the time is generally 1 -10min, the purpose of pre-denaturation is to completely separate the double-stranded nucleotide sequence into single strands; (3) Denaturation, the temperature is generally 90°C-105°C, and the time is generally 10s-30s; (4) Annealing, so that each primer Anneal to the target sequence of human parainfluenza virus type 1, human parainfluenza virus type 2, human parainfluenza virus type 3 or internal standard quality control nucleic acid. The annealing temperature is usually 40°C-60°C, and the annealing time can be 10...
Embodiment 3
[0033] Embodiment 3 Feasibility test of kit of the present invention
[0034] 1. Lower limit of detection (LOD) test
[0035] (1) Preparation of human parainfluenza virus triple nucleic acid detection reagent
[0036] The human parainfluenza virus triple nucleic acid detection reagent was prepared by adopting the method of Example 1.
[0037] (2) Virus sample extraction
[0038] Mix the pharyngeal swab eluate of five different concentrations of human parainfluenza virus type 1, type 2, and type 3 in the tube with a pipette, take out 200 µl into a new centrifuge tube, centrifuge at 12,000 rpm for 5 min, and discard carefully Remove the supernatant; add 200 µl of virus lysate to the pellet, mix well, bathe in water at 100°C for 5 min, and centrifuge at 10,000 rpm for 5 min for later use.
[0039] (3) Sample detection
[0040] Add 25µl of the processed specimen supernatant to the human parainfluenza virus triple nucleic acid detection reaction tube, 20 duplicate wells for eac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com