Aza-tetracene analogue of pyrrole-monoketone and preparation method and application thereof
A technology of pyrrole monoketone and tetracene, which is applied in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., can solve the problems of complex synthesis steps and low yield, and achieve simple synthesis methods, easy purification, high air The effect of stabilizing performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
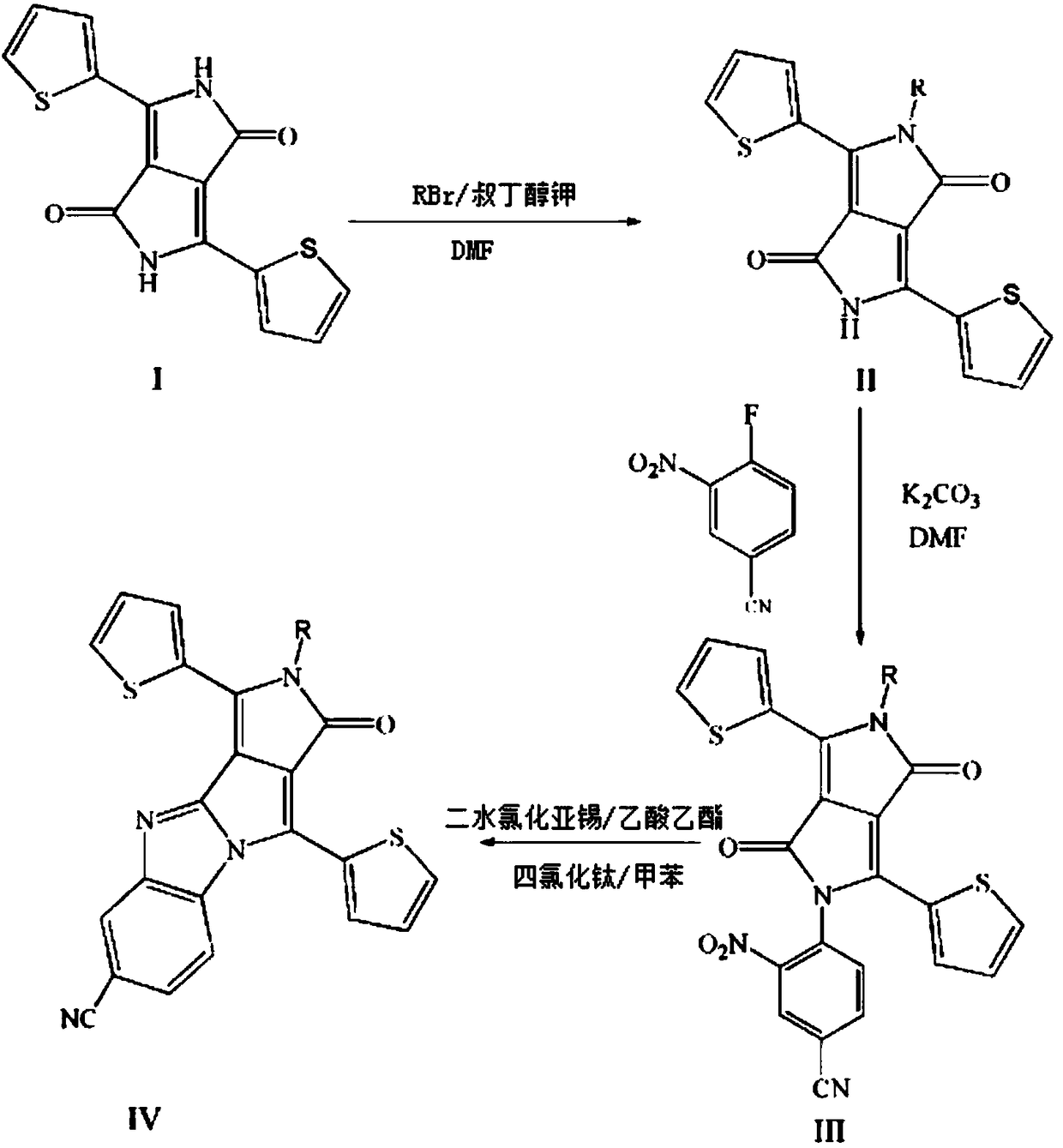
[0067] A method for preparing an aza-tetracene analogue of pyrrole monoketone, comprising the steps of:
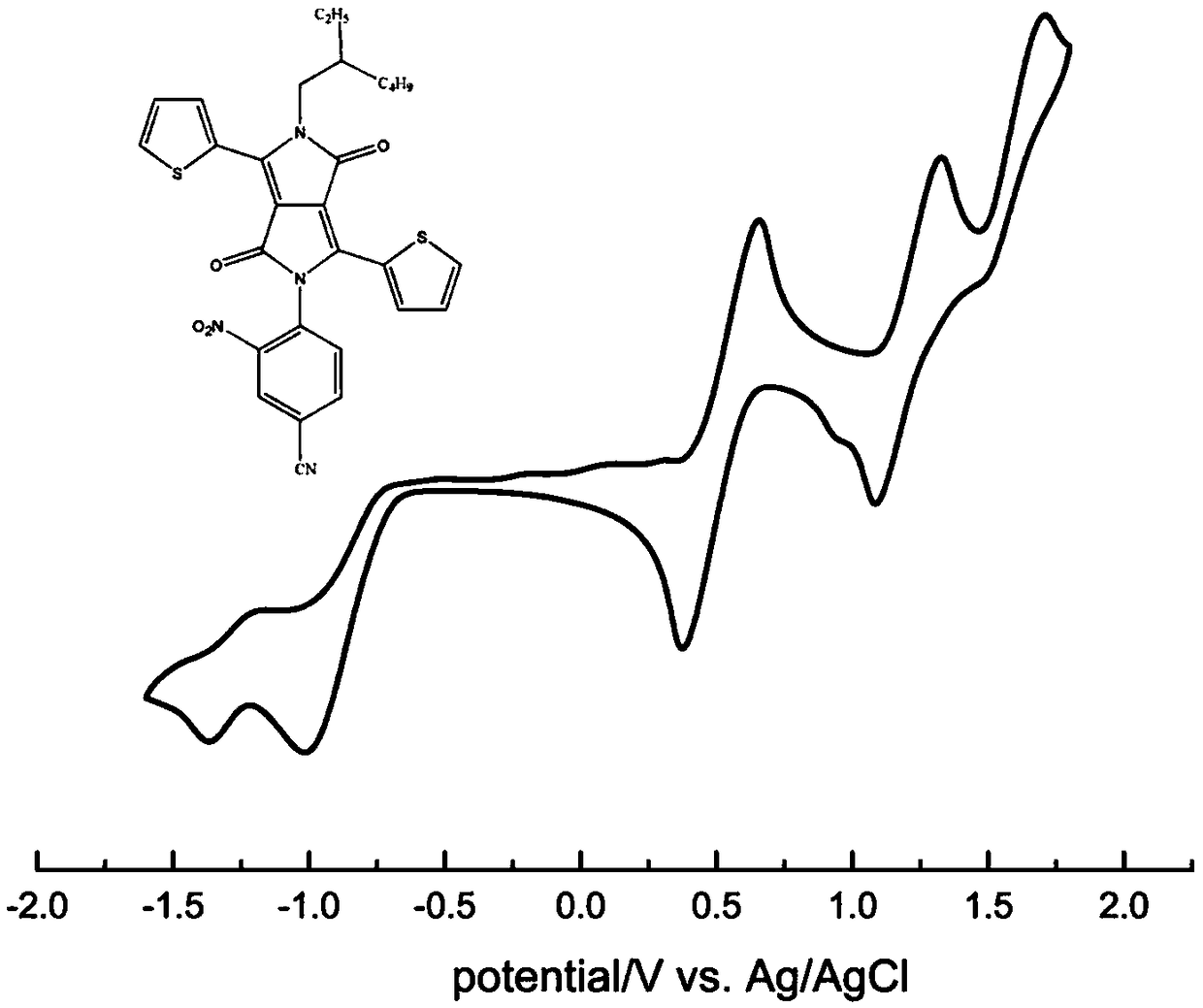
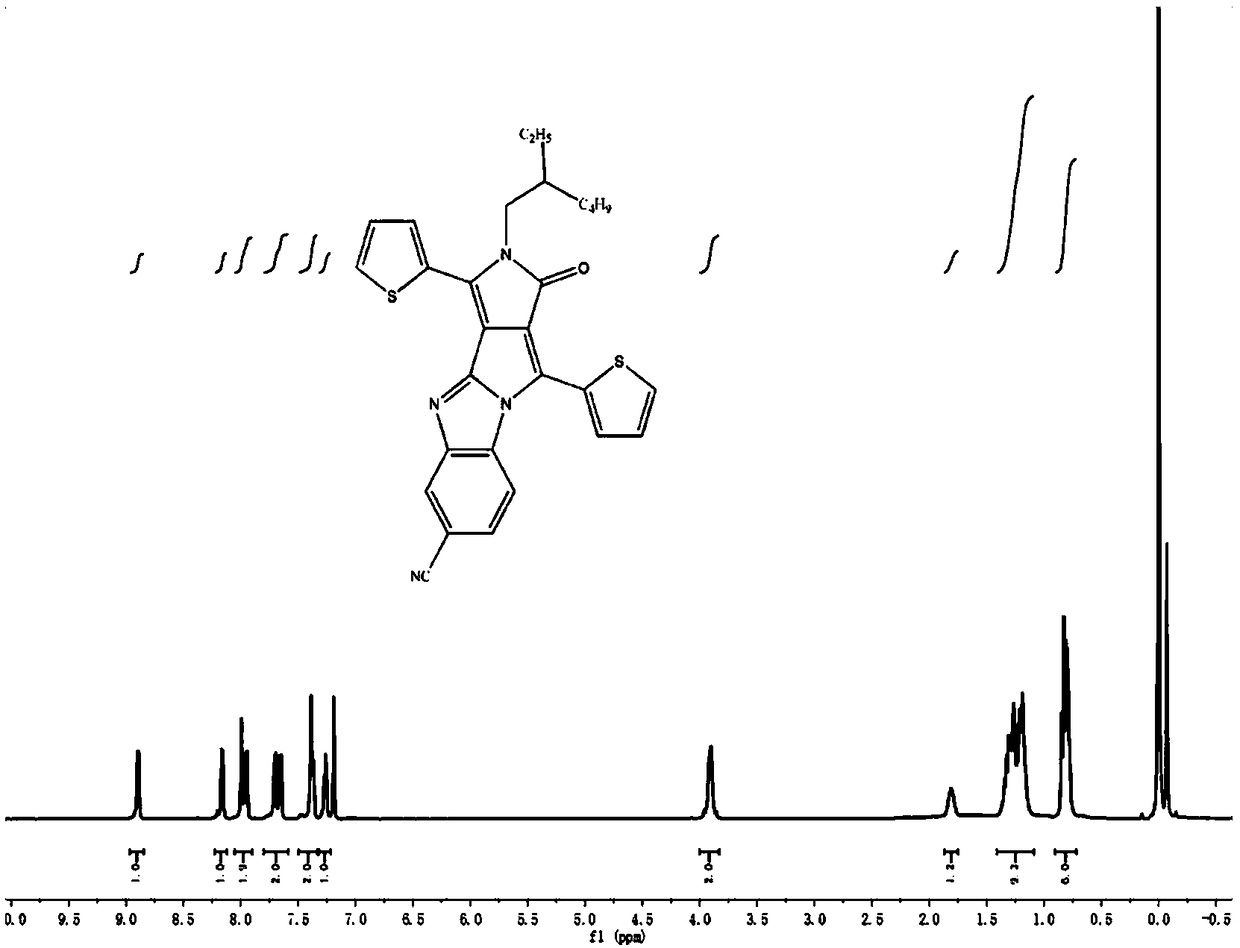
[0068] (1) Mix 3.00 g 3,6-di(2-thienyl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (I) and 2.9 g tert-butanol Potassium was added to a three-necked round-bottomed flask, degassed and then filled with nitrogen for three consecutive times, then added 50 mL N,N-dimethylformamide (DMF), stirred, and then added C 16 h 33 Br (1-bromohexadecane) 4.6 mL, react at room temperature for 4 h. The obtained solution was rotary evaporated first, and then purified by a chromatographic column. The ratio of the eluent dichloromethane to petroleum ether was 1:2. Compound II could be obtained by distillation under reduced pressure. The quality of the obtained compound was 1.57 g. The rate is 30%; figure 2 It is the 1HNMR spectrogram of compound II, and the synthesized compound can be proved to be structure II by hydrogen nuclear magnetic resonance spectrum.
[0069] (2) Add 1.00 g of com...
Embodiment 2
[0072] A method for preparing an aza-tetracene analogue of pyrrole monoketone, comprising the steps of:
[0073] (1) Mix 3.00 g 3,6-di(2-thienyl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (I) and 2.9 g tert-butanol Potassium was added to a three-necked round-bottomed flask, degassed and then filled with nitrogen for three consecutive times, then added 50 mL N,N-dimethylformamide (DMF), stirred, and then added C 7 h 15 Br (bromoisoctane) 2.67 mL, react at room temperature for 4.5 h. The obtained solution was distilled under reduced pressure first, and then purified by a chromatographic column. The ratio of the eluent to dichloromethane and petroleum ether was 1:2. Compound II could be obtained by distillation under reduced pressure, and the quality of the obtained compound was 1.60 g. Yield is 40%; Figure 11 It is the 1H NMR spectrogram of compound II, and the synthesized compound can be proved to be structure II by proton nuclear magnetic resonance spectrum.
[0074] (2)...
Embodiment 3
[0077] (1) Mix 3.00 g 3,6-di(2-thienyl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (I) and 2.9 g tert-butanol Potassium was added to a three-necked round-bottomed flask, degassed and then filled with nitrogen for three consecutive times, then added 50 mL N,N-dimethylformamide (DMF), stirred, and then added C 8 h 17 Br 4.6 mL, react at room temperature for 4 h. The obtained solution was rotary-evaporated first, and then purified by a chromatographic column. The ratio of eluent dichloromethane to petroleum ether was 1:2, and compound II could be obtained by distillation under reduced pressure.
[0078] (2) Add 1.00 g of compound (II), 1.05 g of potassium carbonate and 0.63 g of 3-nitro-4-fluorobenzocyanide into a round bottom flask, then add 40 mL of N,N dimethylformamide, and heat at 80 °C, react for 24 h. , and finally the precipitated solid was filtered and then the solvent was distilled off under reduced pressure, and purified by a chromatography column, wherein the volum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com